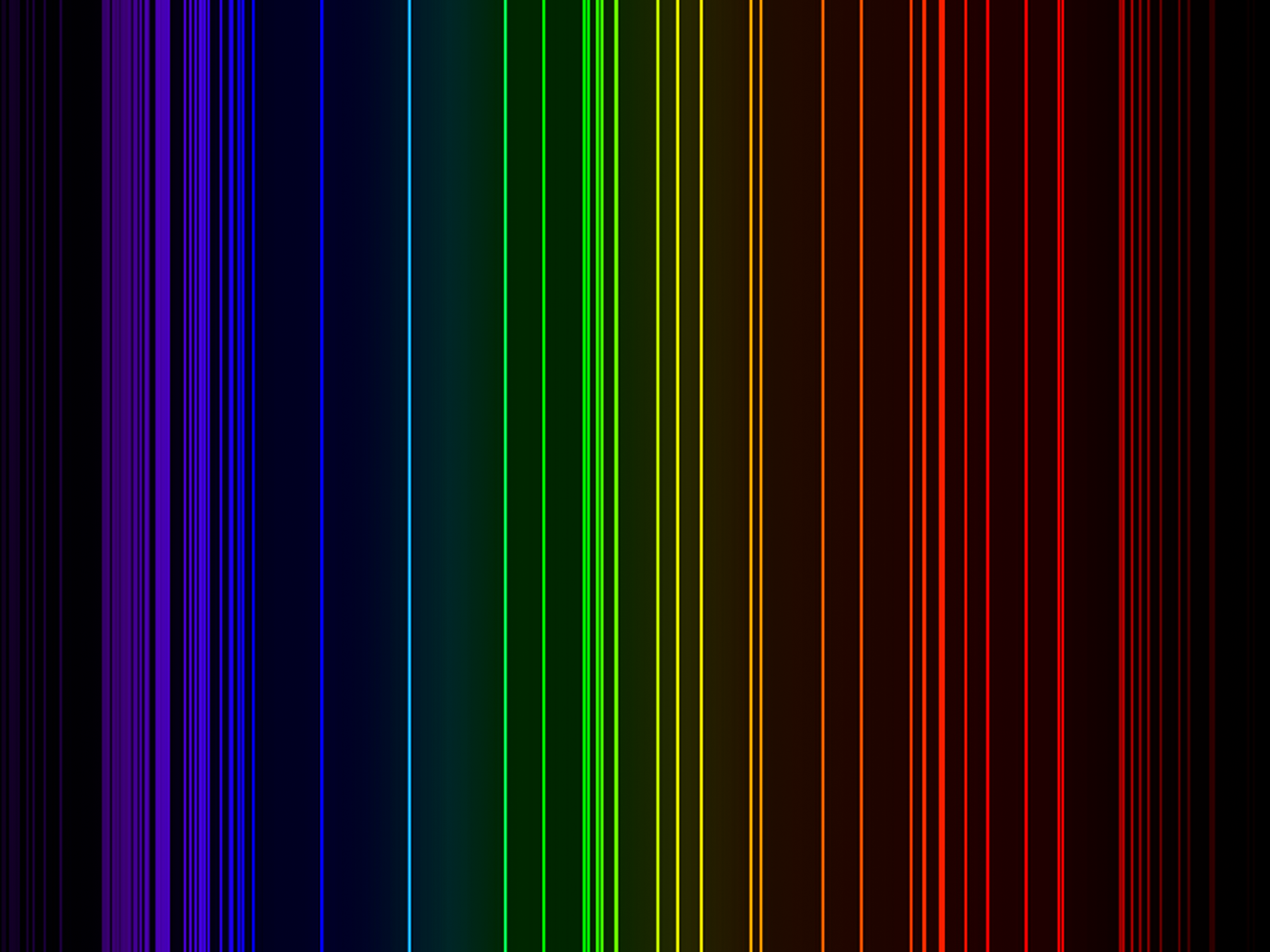
| Isotope_094_pu_228_u |
Unstable |
²²⁸Pu |
Boson |
94 |
p |
134 |
n |
0 |
1 |
228.038'742'328'0 |
u |
~ 0 |
% |
~ 0 |
63.202'000'000'0 |
MeV |
7.514'000'000'0 |
MeV |
- |
|
- |
|
3.17E-10 |
year |
10.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
α |
7,949.600 |
keV |
²²⁴U |
²²⁸Pu > [ 100 % , α , 7,949.6 keV ] > ²²⁴U |
|
|
β+ |
1,365.000 |
keV |
²²⁸Np |
²²⁸Pu > [ , β+ , 1,365.0 keV ] > ²²⁸Np |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.102'404 |
% |
²⁰⁸Pb |
0.000'000 |
% |
²⁰⁶Pb |
? |
% |
²⁰⁰Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_229_u |
Unstable |
²²⁹Pu |
Fermion |
94 |
p |
135 |
n |
3/2 |
1 |
229.040'150'212'0 |
u |
~ 0 |
% |
~ 0 |
36.088'247'000'0 |
MeV |
7.590'489'000'0 |
MeV |
- |
|
- |
|
3.80E-6 |
year |
120.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
α |
7,597.500 |
keV |
²²⁵U |
²²⁹Pu > [ 100 % , α , 7,597.5 keV ] > ²²⁵U |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.480'000 |
% |
²⁰⁵Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_230_u |
Unstable |
²³⁰Pu |
Boson |
94 |
p |
136 |
n |
0 |
1 |
230.039'649'886'0 |
u |
~ 0 |
% |
~ 0 |
37.399'683'000'0 |
MeV |
7.586'862'000'0 |
MeV |
- |
|
- |
|
3.18E-6 |
year |
100.200 |
seconds ( x⁰ ) |
? |
% |
α |
7,179.890 |
keV |
²²⁶U |
²³⁰Pu > [ ? % , α , 7,179.89 keV ] > ²²⁶U |
|
|
β+ |
675.300 |
keV |
²³⁰Np |
²³⁰Pu > [ , β+ , 675.3 keV ] > ²³⁰Np |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
²⁰⁵Tl |
? |
% |
²⁰⁸Pb |
? |
% |
²⁰⁶Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_231_u |
Unstable |
²³¹Pu |
Fermion |
94 |
p |
137 |
n |
3/2 |
1 |
231.041'101'107'0 |
u |
~ 0 |
% |
~ 0 |
36.933'632'000'0 |
MeV |
7.590'994'000'0 |
MeV |
- |
|
- |
|
1.65E-5 |
year |
520.020 |
seconds ( x⁰ ) |
87.000'000 |
% |
β+ |
1,638.200 |
keV |
²³¹Np |
²³¹Pu > [ 87 % , β+ , 1,638.2 keV ] > ²³¹Np |
|
|
α |
6,838.500 |
keV |
²²⁷U |
²³¹Pu > [ , α , 6,838.5 keV ] > ²²⁷U |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.243'938 |
% |
²⁰⁷Pb |
0.000'000 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁸Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_232_u |
Unstable |
²³²Pu |
Boson |
94 |
p |
138 |
n |
0 |
1 |
232.041'187'097'0 |
u |
~ 0 |
% |
~ 0 |
38.285'435'000'0 |
MeV |
7.587'222'000'0 |
MeV |
- |
|
- |
|
6.40E-5 |
year |
2.020 |
kilo-seconds ( x³ ) |
89.000'000 |
% |
ϵ |
1,005.000 |
keV |
²³²Np |
²³²Pu > [ 89 % , ϵ , 1,005.0 keV ] > ²³²Np |
|
|
α |
6,715.900 |
keV |
²²⁸U |
²³²Pu > [ , α , 6,715.9 keV ] > ²²⁸U |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.006'433 |
% |
²⁰⁸Pb |
0.000'000 |
% |
²⁰⁶Pb |
? |
% |
²⁰⁰Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_233_u |
Unstable |
²³³Pu |
Fermion |
94 |
p |
139 |
n |
5/2 |
1 |
233.042'997'375'0 |
u |
~ 0 |
% |
~ 0 |
38.365'534'000'0 |
MeV |
7.588'963'000'0 |
MeV |
- |
|
- |
|
3.96E-5 |
year |
1.250 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
1,080.000 |
keV |
²³³Np |
²³³Pu > [ 100 % , β+ , 1,080.0 keV ] > ²³³Np |
|
|
α |
6,416.300 |
keV |
²²⁹U |
²³³Pu > [ , α , 6,416.3 keV ] > ²²⁹U |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.134'278 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁷Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_234_u |
Unstable |
²³⁴Pu |
Boson |
94 |
p |
140 |
n |
0 |
1 |
234.043'317'076'0 |
u |
~ 0 |
% |
~ 0 |
40.051'797'000'0 |
MeV |
7.584'7.584'7.584'7.584 |
MeV |
- |
|
- |
|
1.69E-5 |
year |
533.400 |
seconds ( x⁰ ) |
94.000'000 |
% |
ϵ |
393.100 |
keV |
²³⁴Np |
²³⁴Pu > [ 94 % , ϵ , 393.1 keV ] > ²³⁴Np |
|
|
α |
6,309.980 |
keV |
²³⁰U |
²³⁴Pu > [ , α , 6,309.98 keV ] > ²³⁰U |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.041'517 |
% |
²⁰⁶Pb |
0.000'002 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁸Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_235_u |
Unstable |
²³⁵Pu |
Fermion |
94 |
p |
141 |
n |
5/2 |
1 |
235.045'286'050'0 |
u |
~ 0 |
% |
~ 0 |
40.349'597'000'0 |
MeV |
7.584'607'000'0 |
MeV |
- |
|
- |
|
4.82E-5 |
year |
1.520 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
116.800 |
keV |
²³⁵Np |
²³⁵Pu > [ 100 % , β+ , 116.8 keV ] > ²³⁵Np |
|
|
α |
5,951.400 |
keV |
²³¹U |
²³⁵Pu > [ , α , 5,951.4 keV ] > ²³¹U |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.281'729 |
% |
²⁰⁷Pb |
0.000'000 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁶Pb |
? |
% |
¹⁸⁰Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_236_u |
Unstable |
²³⁶Pu |
Boson |
94 |
p |
142 |
n |
0 |
1 |
236.046'057'964'0 |
u |
~ 0 |
% |
~ 0 |
42.183'684'000'0 |
MeV |
7.578'873'000'0 |
MeV |
- |
|
- |
|
2.86E+0 |
years |
90.252 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
α |
5,867.070 |
keV |
²³²U |
²³⁶Pu > [ 100 % , α , 5,867.07 keV ] > ²³²U |
|
|
SF |
? |
keV |
V |
²³⁶Pu > [ , SF , ? keV ] > V |
0.000'000 |
% |
²⁸Mg |
? |
keV |
²⁰⁸Pb |
²³⁶Pu > [ 0.000000000002 % , ²⁸Mg , ? keV ] > ²⁰⁸Pb |
? |
% |
2β+ |
-1,588.010 |
keV |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.004'000 |
% |
²⁰⁸Pb |
0.000'000 |
% |
²⁰⁶Pb |
? |
% |
²⁰⁰Hg |
0.000'000 |
% |
²⁰⁸Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_237_u |
Unstable |
²³⁷Pu |
Fermion |
94 |
p |
143 |
n |
7/2 |
-1 |
237.048'409'658'0 |
u |
~ 0 |
% |
~ 0 |
42.902'718'000'0 |
MeV |
7.577'913'000'0 |
MeV |
- |
|
- |
|
1.24E-1 |
year |
3.910 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
ϵ |
200.030 |
keV |
²³⁷Np |
²³⁷Pu > [ 100 % , ϵ , 200.03 keV ] > ²³⁷Np |
|
|
α |
5,748.430 |
keV |
²³³U |
²³⁷Pu > [ , α , 5,748.43 keV ] > ²³³U |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.017'001 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁷Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_238_u |
Unstable |
²³⁸Pu |
Boson |
94 |
p |
144 |
n |
0 |
1 |
238.049'559'894'0 |
u |
~ 0 |
% |
~ 0 |
45.093'307'000'0 |
MeV |
7.570'752'000'0 |
MeV |
- |
|
- |
|
8.78E+1 |
years |
2.772 |
giga-seconds ( x⁹ ) |
100.000'000 |
% |
α |
5,593.200 |
keV |
²³⁴U |
²³⁸Pu > [ 100 % , α , 5,593.2 keV ] > ²³⁴U |
|
|
SF |
? |
keV |
V |
²³⁸Pu > [ , SF , ? keV ] > V |
0.000'000 |
% |
³²Si |
? |
keV |
²⁰⁶Hg |
²³⁸Pu > [ 0.000000000000014 % , ³²Si , ? keV ] > ²⁰⁶Hg |
0.000'000 |
% |
|
? |
keV |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.044'159 |
% |
²⁰⁶Pb |
0.000'002 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁸Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_239_u |
Unstable |
²³⁹Pu |
Fermion |
94 |
p |
145 |
n |
1/2 |
1 |
239.052'163'381'0 |
u |
~ 0 |
% |
~ 0 |
46.164'745'000'0 |
MeV |
7.568'354'000'0 |
MeV |
0.203'000'000'0 |
nm |
- |
|
2.41E+4 |
years |
761.327 |
giga-seconds ( x⁹ ) |
100.000'000 |
% |
α |
5,244.510 |
keV |
²³⁵U |
²³⁹Pu > [ 100 % , α , 5,244.51 keV ] > ²³⁵U |
|
|
SF |
? |
keV |
V |
²³⁹Pu > [ , SF , ? keV ] > V |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.276'314 |
% |
²⁰⁷Pb |
0.000'000 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁶Pb |
0.000'000 |
% |
¹⁶⁰Dy |
0.000'000 |
% |
¹⁴⁰Ce |
? |
% |
¹⁷²Yb |
? |
% |
¹⁷⁶Hf |
? |
% |
¹⁵²Sm |
? |
% |
¹⁸⁰Hf |
? |
% |
¹⁵⁶Gd |
? |
% |
¹⁶⁸Er |
? |
% |
¹⁶⁴Dy |
| Isotope_094_pu_240_u |
Unstable |
²⁴⁰Pu |
Boson |
94 |
p |
146 |
n |
0 |
1 |
240.053'813'545'0 |
u |
~ 0 |
% |
~ 0 |
48.589'877'000'0 |
MeV |
7.560'311'000'0 |
MeV |
- |
|
- |
|
6.57E+3 |
years |
207.242 |
giga-seconds ( x⁹ ) |
100.000'000 |
% |
α |
5,255.750 |
keV |
²³⁶U |
²⁴⁰Pu > [ 100 % , α , 5,255.75 keV ] > ²³⁶U |
|
|
SF |
? |
keV |
V |
²⁴⁰Pu > [ , SF , ? keV ] > V |
0.000'000 |
% |
³⁴Si |
? |
keV |
²⁰⁶Hg |
²⁴⁰Pu > [ 0.00000000000013 % , ³⁴Si , ? keV ] > ²⁰⁶Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.004'000 |
% |
²⁰⁸Pb |
0.000'000 |
% |
²⁰⁶Pb |
? |
% |
²⁰⁰Hg |
0.000'000 |
% |
²⁰⁸Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_241_u |
Unstable |
²⁴¹Pu |
Fermion |
94 |
p |
147 |
n |
5/2 |
1 |
241.056'851'456'0 |
u |
~ 0 |
% |
~ 0 |
50.126'995'000'0 |
MeV |
7.556'036'000'0 |
MeV |
-0.683'000'000'0 |
nm |
- |
|
1.44E+1 |
years |
453.104 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
β- |
20.783 |
keV |
²⁴¹Am |
²⁴¹Pu > [ 100 % , β- , 20.783 keV ] > ²⁴¹Am |
|
|
α |
5,140.000 |
keV |
²³⁷U |
²⁴¹Pu > [ , α , 5,140.0 keV ] > ²³⁷U |
0.000'000 |
% |
SF |
? |
keV |
V |
²⁴¹Pu > [ 0.000000000000024 % , SF , ? keV ] > V |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.015'250 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁷Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_242_u |
Unstable |
²⁴²Pu |
Boson |
94 |
p |
148 |
n |
0 |
1 |
242.058'742'611'0 |
u |
~ 0 |
% |
~ 0 |
52.956'791'000'0 |
MeV |
7.546'432'000'0 |
MeV |
- |
|
5.600'000'000'0 |
b |
3.74E+5 |
years |
11.809 |
tera-seconds ( x¹² ) |
100.000'000 |
% |
α |
4,984.530 |
keV |
²³⁸U |
²⁴²Pu > [ 100 % , α , 4,984.53 keV ] > ²³⁸U |
|
|
SF |
? |
keV |
V |
²⁴²Pu > [ , SF , ? keV ] > V |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.044'159 |
% |
²⁰⁶Pb |
0.000'002 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁸Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_243_u |
Unstable |
²⁴³Pu |
Fermion |
94 |
p |
149 |
n |
7/2 |
1 |
243.062'003'092'0 |
u |
~ 0 |
% |
~ 0 |
54.718'390'000'0 |
MeV |
7.541'321'000'0 |
MeV |
- |
|
- |
|
5.65E-4 |
year |
17.840 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
579.400 |
keV |
²⁴³Am |
²⁴³Pu > [ 100 % , β- , 579.4 keV ] > ²⁴³Am |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.276'314 |
% |
²⁰⁷Pb |
0.000'000 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁶Pb |
0.000'000 |
% |
¹⁶⁰Dy |
0.000'000 |
% |
¹⁴⁰Ce |
? |
% |
¹⁷²Yb |
? |
% |
¹⁷⁶Hf |
? |
% |
¹⁵²Sm |
? |
% |
¹⁸⁰Hf |
? |
% |
¹⁵⁶Gd |
? |
% |
¹⁶⁸Er |
? |
% |
¹⁶⁴Dy |
| Isotope_094_pu_244_u |
Unstable |
²⁴⁴Pu |
Boson |
94 |
p |
150 |
n |
0 |
1 |
244.064'203'907'0 |
u |
~ 0 |
% |
~ 0 |
57.755'509'000'0 |
MeV |
7.531'004'000'0 |
MeV |
- |
|
- |
|
7.93E+7 |
years |
2.503 |
peta-seconds ( x¹⁵ ) |
100.000'000 |
% |
α |
4,665.540 |
keV |
²⁴⁰U |
²⁴⁴Pu > [ 100 % , α , 4,665.54 keV ] > ²⁴⁰U |
|
|
SF |
? |
keV |
V |
²⁴⁴Pu > [ , SF , ? keV ] > V |
0.000'000 |
% |
2β- |
1,351.900 |
keV |
²⁴⁴Cm |
²⁴⁴Pu > [ 0.0000000073 % , 2β- , 1,351.9 keV ] > ²⁴⁴Cm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.004'000 |
% |
²⁰⁸Pb |
0.000'000 |
% |
²⁰⁶Pb |
? |
% |
²⁰⁰Hg |
0.000'000 |
% |
²⁰⁸Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_245_u |
Unstable |
²⁴⁵Pu |
Fermion |
94 |
p |
151 |
n |
9/2 |
-1 |
245.067'747'154'0 |
u |
~ 0 |
% |
~ 0 |
59.805'555'000'0 |
MeV |
7.524'817'000'0 |
MeV |
- |
|
- |
|
1.20E-3 |
year |
37.800 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
1,206.300 |
keV |
²⁴⁵Am |
²⁴⁵Pu > [ 100 % , β- , 1,206.3 keV ] > ²⁴⁵Am |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.015'250 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁷Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_246_u |
Unstable |
²⁴⁶Pu |
Boson |
94 |
p |
152 |
n |
0 |
1 |
246.070'204'627'0 |
u |
~ 0 |
% |
~ 0 |
63.106'068'000'0 |
MeV |
7.513'576'000'0 |
MeV |
- |
|
- |
|
1.24E-3 |
year |
39.024 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
400.500 |
keV |
²⁴⁶Am |
²⁴⁶Pu > [ 100 % , β- , 400.5 keV ] > ²⁴⁶Am |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.044'159 |
% |
²⁰⁶Pb |
0.000'002 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁸Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_094_pu_247_u |
Unstable |
²⁴⁷Pu |
Fermion |
94 |
p |
153 |
n |
1/2 |
1 |
247.074'070'000'0 |
u |
~ 0 |
% |
~ 0 |
65.395'190'000'0 |
MeV |
7.506'538'000'0 |
MeV |
- |
|
- |
|
6.21E-3 |
year |
196.042 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
1,842.000 |
keV |
²⁴⁷Am |
²⁴⁷Pu > [ 100 % , β- , 1,842.0 keV ] > ²⁴⁷Am |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.276'314 |
% |
²⁰⁷Pb |
0.000'000 |
% |
²⁰⁵Tl |
0.000'000 |
% |
²⁰⁶Pb |
0.000'000 |
% |
¹⁶⁰Dy |
0.000'000 |
% |
¹⁴⁰Ce |
? |
% |
¹⁷²Yb |
? |
% |
¹⁷⁶Hf |
? |
% |
¹⁵²Sm |
? |
% |
¹⁸⁰Hf |
? |
% |
¹⁵⁶Gd |
? |
% |
¹⁶⁸Er |
? |
% |
¹⁶⁴Dy |