| Isotope_082_pb_178_u |
Unstable |
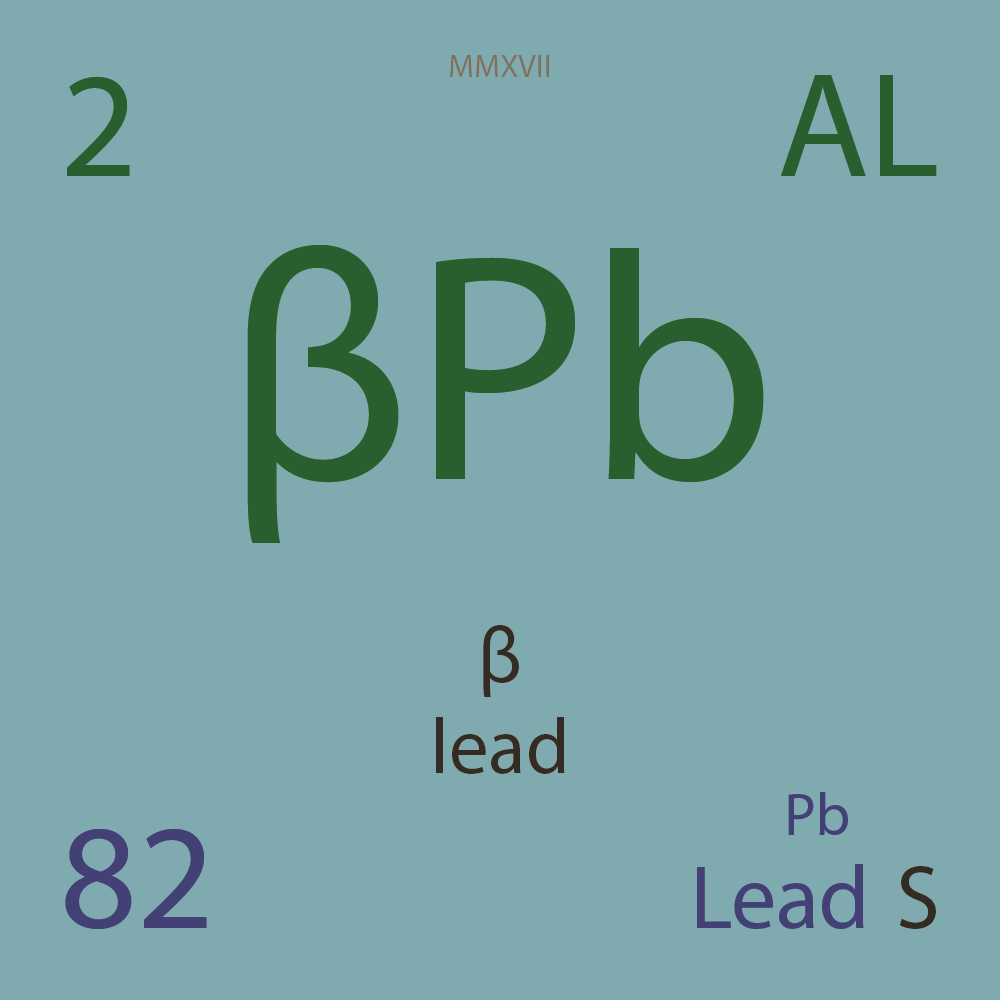
¹⁷⁸Pb |
Boson |
82 |
p |
96 |
n |
0 |
1 |
178.003'830'191'0 |
u |
~ 0 |
% |
~ 0 |
3.567'800'000'0 |
MeV |
7.690'866'000'0 |
MeV |
- |
|
- |
|
7.29E-12 |
year |
230.000 |
micro-seconds ( x⁻⁶ ) |
100.000'000 |
% |
α |
7,790.300 |
keV |
¹⁷⁴Hg |
¹⁷⁸Pb > [ 100 % , α , 7,790.3 keV ] > ¹⁷⁴Hg |
|
|
β+ |
7,299.000 |
keV |
¹⁷⁸Tl |
¹⁷⁸Pb > [ , β+ , 7,299.0 keV ] > ¹⁷⁸Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
14.519'370 |
% |
¹⁴²Nd |
2.044'874 |
% |
¹⁷⁰Yb |
0.213'059 |
% |
¹⁶⁶Er |
? |
% |
¹⁷⁸Hf |
? |
% |
¹⁵⁴Gd |
? |
% |
¹⁷⁴Yb |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁸Gd |
? |
% |
¹⁶²Dy |
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_179_u |
Unstable |
¹⁷⁹Pb |
Fermion |
82 |
p |
97 |
n |
5/2 |
-1 |
179.002'150'000'0 |
u |
~ 0 |
% |
~ 0 |
2.003'000'000'0 |
MeV |
7.702'000'000'0 |
MeV |
- |
|
- |
|
9.51E-11 |
year |
3.000 |
milli-seconds ( x⁻³ ) |
? |
% |
α |
7,567.000 |
keV |
¹⁷⁵Hg |
¹⁷⁹Pb > [ ? % , α , 7,567.0 keV ] > ¹⁷⁵Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁷¹Yb |
? |
% |
¹⁵⁹Tb |
? |
% |
¹⁴³Nd |
? |
% |
¹⁷⁵Lu |
? |
% |
¹⁵⁵Gd |
? |
% |
¹⁵¹Eu |
? |
% |
¹⁶⁷Er |
? |
% |
¹⁶³Dy |
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_180_u |
Unstable |
¹⁸⁰Pb |
Boson |
82 |
p |
98 |
n |
0 |
1 |
179.997'918'173'0 |
u |
~ 0 |
% |
~ 0 |
-1.939'209'000'0 |
MeV |
7.725'688'000'0 |
MeV |
- |
|
- |
|
1.58E-10 |
year |
5.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
α |
7,415.000 |
keV |
¹⁷⁶Hg |
¹⁸⁰Pb > [ 100 % , α , 7,415.0 keV ] > ¹⁷⁶Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.281'333 |
% |
¹⁶⁰Dy |
0.000'084 |
% |
¹⁴⁰Ce |
? |
% |
¹⁷²Yb |
? |
% |
¹⁷⁶Hf |
? |
% |
¹⁵²Sm |
? |
% |
¹⁵⁶Gd |
? |
% |
¹⁶⁸Er |
? |
% |
¹⁶⁴Dy |
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_181_u |
Unstable |
¹⁸¹Pb |
Fermion |
82 |
p |
99 |
n |
5/2 |
-1 |
180.996'623'958'0 |
u |
~ 0 |
% |
~ 0 |
-3.144'762'000'0 |
MeV |
7.734'258'000'0 |
MeV |
- |
|
- |
|
1.43E-9 |
year |
45.000 |
milli-seconds ( x⁻³ ) |
98.000'000 |
% |
α |
7,211.000 |
keV |
¹⁷⁷Hg |
¹⁸¹Pb > [ 98 % , α , 7,211.0 keV ] > ¹⁷⁷Hg |
|
|
β+ |
8,634.100 |
keV |
¹⁸¹Tl |
¹⁸¹Pb > [ , β+ , 8,634.1 keV ] > ¹⁸¹Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
64.338'092 |
% |
¹⁶⁹Tm |
26.066'040 |
% |
¹⁷³Yb |
7.700'132 |
% |
¹⁶⁵Ho |
0.015'394 |
% |
¹⁶¹Dy |
0.000'020 |
% |
¹⁵⁷Gd |
0.000'000 |
% |
¹⁵³Eu |
0.000'000 |
% |
¹⁴⁵Nd |
0.000'000 |
% |
¹⁴¹Pr |
? |
% |
¹⁷⁷Hf |
? |
% |
¹⁷⁶Hf |
? |
% |
¹⁸⁰Hf |
|
|
|
| Isotope_082_pb_182_u |
Unstable |
¹⁸²Pb |
Boson |
82 |
p |
100 |
n |
0 |
1 |
181.992'671'842'0 |
u |
~ 0 |
% |
~ 0 |
-6.826'135'000'0 |
MeV |
7.756'338'000'0 |
MeV |
- |
|
- |
|
1.90E-9 |
year |
60.000 |
milli-seconds ( x⁻³ ) |
98.000'000 |
% |
α |
7,065.800 |
keV |
¹⁷⁸Hg |
¹⁸²Pb > [ 98 % , α , 7,065.8 keV ] > ¹⁷⁸Hg |
|
|
β+ |
5,502.700 |
keV |
¹⁸²Tl |
¹⁸²Pb > [ , β+ , 5,502.7 keV ] > ¹⁸²Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
60.829'830 |
% |
¹⁷⁰Yb |
16.573'363 |
% |
¹⁷⁸Hf |
4.965'611 |
% |
¹⁶⁶Er |
0.000'000 |
% |
¹⁸¹Ta |
0.000'000 |
% |
¹⁷⁷Hf |
0.000'000 |
% |
¹⁴²Nd |
? |
% |
¹⁵⁴Gd |
? |
% |
¹⁷⁴Yb |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁸Gd |
? |
% |
¹⁶²Dy |
|
|
|
| Isotope_082_pb_183_u |
Unstable |
¹⁸³Pb |
Fermion |
82 |
p |
101 |
n |
3/2 |
-1 |
182.991'874'629'0 |
u |
~ 0 |
% |
~ 0 |
-7.568'734'000'0 |
MeV |
7.762'117'000'0 |
MeV |
- |
|
- |
|
1.70E-8 |
year |
535.000 |
milli-seconds ( x⁻³ ) |
90.000'000 |
% |
α |
6,928.000 |
keV |
¹⁷⁹Hg |
¹⁸³Pb > [ 90 % , α , 6,928.0 keV ] > ¹⁷⁹Hg |
|
|
β+ |
7,996.400 |
keV |
¹⁸²Tl |
¹⁸³Pb > [ , β+ , 7,996.4 keV ] > ¹⁸²Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
29.978'870 |
% |
¹⁷¹Yb |
1.355'351 |
% |
¹⁷⁹Hf |
0.549'284 |
% |
¹⁶⁷Er |
0.124'605 |
% |
¹⁷⁸Hf |
0.046'752 |
% |
¹⁷⁵Lu |
0.010'397 |
% |
¹⁷⁰Yb |
0.000'220 |
% |
¹⁶³Dy |
0.000'000 |
% |
¹⁶⁶Er |
0.000'000 |
% |
¹⁵⁹Tb |
? |
% |
¹⁷⁴Yb |
|
|
|
|
|
|
| Isotope_082_pb_184_u |
Unstable |
¹⁸⁴Pb |
Boson |
82 |
p |
102 |
n |
0 |
1 |
183.988'142'339'0 |
u |
~ 0 |
% |
~ 0 |
-11.045'339'000'0 |
MeV |
7.782'692'000'0 |
MeV |
- |
|
- |
|
1.55E-8 |
year |
490.000 |
milli-seconds ( x⁻³ ) |
80.000'000 |
% |
α |
6,774.470 |
keV |
¹⁸⁰Hg |
¹⁸⁴Pb > [ 80 % , α , 6,774.47 keV ] > ¹⁸⁰Hg |
|
|
β+ |
4,817.500 |
keV |
¹⁸⁴Tl |
¹⁸⁴Pb > [ , β+ , 4,817.5 keV ] > ¹⁸⁴Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23.918'093 |
% |
¹⁷⁶Hf |
15.192'749 |
% |
¹⁷²Yb |
? |
% |
¹⁸⁰Hf |
? |
% |
¹⁶⁸Er |
? |
% |
¹⁶⁴Dy |
? |
% |
¹⁶⁰Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_185_u |
Unstable |
¹⁸⁵Pb |
Fermion |
82 |
p |
103 |
n |
3/2 |
-1 |
184.987'609'944'0 |
u |
~ 0 |
% |
~ 0 |
-11.541'263'000'0 |
MeV |
7.786'932'000'0 |
MeV |
- |
|
- |
|
2.00E-7 |
year |
6.300 |
seconds ( x⁰ ) |
50.000'000 |
% |
α |
6,695.000 |
keV |
¹⁸¹Hg |
¹⁸⁵Pb > [ 50 % , α , 6,695.0 keV ] > ¹⁸¹Hg |
|
|
β+ |
7,192.300 |
keV |
¹⁸⁵Tl |
¹⁸⁵Pb > [ , β+ , 7,192.3 keV ] > ¹⁸⁵Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
14.616'505 |
% |
¹⁷⁷Hf |
0.892'270 |
% |
¹⁷³Yb |
0.003'534 |
% |
¹⁶⁹Tm |
0.000'024 |
% |
¹⁷⁶Hf |
? |
% |
¹⁸¹Ta |
? |
% |
¹⁸⁵Re |
? |
% |
¹⁸⁰Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_186_u |
Unstable |
¹⁸⁶Pb |
Boson |
82 |
p |
104 |
n |
0 |
1 |
185.984'238'945'0 |
u |
~ 0 |
% |
~ 0 |
-14.681'328'000'0 |
MeV |
7.805'7.805'7.805'7.805 |
MeV |
- |
|
- |
|
1.53E-7 |
year |
4.820 |
seconds ( x⁰ ) |
60.000'000 |
% |
β+ |
4,487.000 |
keV |
¹⁸⁶Tl |
¹⁸⁶Pb > [ 60 % , β+ , 4,487.0 keV ] > ¹⁸⁶Tl |
|
|
α |
6,469.900 |
keV |
¹⁸²Hg |
¹⁸⁶Pb > [ , α , 6,469.9 keV ] > ¹⁸²Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5.152'886 |
% |
¹⁷⁸Hf |
5.152'886 |
% |
¹⁷⁰Yb |
0.000'004 |
% |
¹⁸¹Ta |
0.000'001 |
% |
¹⁶⁶Er |
0.000'000 |
% |
¹⁷⁷Hf |
? |
% |
¹⁷⁴Yb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_187_u |
Unstable |
¹⁸⁷Pb |
Fermion |
82 |
p |
105 |
n |
3/2 |
-1 |
186.983'918'370'0 |
u |
~ 0 |
% |
~ 0 |
-14.979'941'000'0 |
MeV |
7.808'363'000'0 |
MeV |
- |
|
- |
|
4.82E-7 |
year |
15.200 |
seconds ( x⁰ ) |
93.000'000 |
% |
β+ |
6,441.400 |
keV |
¹⁸⁷Tl |
¹⁸⁷Pb > [ 93 % , β+ , 6,441.4 keV ] > ¹⁸⁷Tl |
|
|
α |
6,394.960 |
keV |
¹⁸³Hg |
¹⁸⁷Pb > [ , α , 6,394.96 keV ] > ¹⁸³Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
93.000'000 |
% |
¹⁸⁷Os |
0.856'681 |
% |
¹⁷⁹Hf |
0.001'966 |
% |
¹⁷⁵Lu |
0.000'000 |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_188_u |
Unstable |
¹⁸⁸Pb |
Boson |
82 |
p |
106 |
n |
0 |
1 |
187.980'874'338'0 |
u |
~ 0 |
% |
~ 0 |
-17.815'439'000'0 |
MeV |
7.824'844'000'0 |
MeV |
- |
|
- |
|
8.08E-7 |
year |
25.500 |
seconds ( x⁰ ) |
90.700'000 |
% |
β+ |
3,509.100 |
keV |
¹⁸⁸Tl |
¹⁸⁸Pb > [ 90.7 % , β+ , 3,509.1 keV ] > ¹⁸⁸Tl |
|
|
α |
6,108.770 |
keV |
¹⁸⁴Hg |
¹⁸⁸Pb > [ , α , 6,108.77 keV ] > ¹⁸⁴Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
90.700'000 |
% |
¹⁸⁸Os |
0.000'310 |
% |
¹⁷⁶Hf |
? |
% |
¹⁸⁰Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_189_u |
Unstable |
¹⁸⁹Pb |
Fermion |
82 |
p |
107 |
n |
3/2 |
-1 |
188.980'807'000'0 |
u |
~ 0 |
% |
~ 0 |
-17.878'165'000'0 |
MeV |
7.826'480'000'0 |
MeV |
- |
|
- |
|
1.62E-6 |
year |
51.000 |
seconds ( x⁰ ) |
99.000'000 |
% |
β+ |
5,701.900 |
keV |
¹⁸⁹Tl |
¹⁸⁹Pb > [ 99 % , β+ , 5,701.9 keV ] > ¹⁸⁹Tl |
|
|
α |
5,872.800 |
keV |
¹⁸⁵Hg |
¹⁸⁹Pb > [ , α , 5,872.8 keV ] > ¹⁸⁵Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
99.000'000 |
% |
¹⁸⁹Tl |
0.376'059 |
% |
¹⁸⁵Re |
0.024'996 |
% |
¹⁸¹Ta |
0.000'018 |
% |
¹⁷⁷Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_190_u |
Unstable |
¹⁹⁰Pb |
Boson |
82 |
p |
108 |
n |
0 |
1 |
189.978'081'517'0 |
u |
~ 0 |
% |
~ 0 |
-20.416'935'000'0 |
MeV |
7.841'130'000'0 |
MeV |
- |
|
- |
|
2.24E-6 |
year |
70.800 |
seconds ( x⁰ ) |
99.600'000 |
% |
β+ |
2,894.100 |
keV |
¹⁹⁰Tl |
¹⁹⁰Pb > [ 99.6 % , β+ , 2,894.1 keV ] > ¹⁹⁰Tl |
|
|
α |
5,697.460 |
keV |
¹⁸⁶Hg |
¹⁹⁰Pb > [ , α , 5,697.46 keV ] > ¹⁸⁶Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.000'000 |
% |
¹⁷⁸Hf |
? |
% |
¹⁹⁰Os |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_191_u |
Unstable |
¹⁹¹Pb |
Fermion |
82 |
p |
109 |
n |
3/2 |
-1 |
190.978'265'000'0 |
u |
~ 0 |
% |
~ 0 |
-20.246'022'000'0 |
MeV |
7.841'441'000'0 |
MeV |
- |
|
- |
|
2.53E-6 |
year |
79.800 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
5,012.800 |
keV |
¹⁹¹Tl |
¹⁹¹Pb > [ 100 % , β+ , 5,012.8 keV ] > ¹⁹¹Tl |
|
|
α |
5,446.900 |
keV |
¹⁸⁷Hg |
¹⁹¹Pb > [ , α , 5,446.9 keV ] > ¹⁸⁷Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.013'000 |
% |
¹⁸⁷Os |
0.000'000 |
% |
¹⁷⁹Hf |
? |
% |
¹⁹¹Ir |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_192_u |
Unstable |
¹⁹²Pb |
Boson |
82 |
p |
110 |
n |
0 |
1 |
191.975'785'171'0 |
u |
~ 0 |
% |
~ 0 |
-22.555'968'000'0 |
MeV |
7.854'669'000'0 |
MeV |
- |
|
- |
|
6.65E-6 |
year |
210.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
2,294.100 |
keV |
¹⁹²Tl |
¹⁹²Pb > [ 100 % , β+ , 2,294.1 keV ] > ¹⁹²Tl |
|
|
α |
5,220.900 |
keV |
¹⁸⁸Hg |
¹⁹²Pb > [ , α , 5,220.9 keV ] > ¹⁸⁸Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹²Pt |
0.005'904 |
% |
¹⁸⁸Os |
? |
% |
¹⁸⁰Hf |
? |
% |
¹⁷⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_193_u |
Unstable |
¹⁹³Pb |
Fermion |
82 |
p |
111 |
n |
3/2 |
-1 |
192.976'173'234'0 |
u |
~ 0 |
% |
~ 0 |
-22.194'490'000'0 |
MeV |
7.853'918'000'0 |
MeV |
- |
|
- |
|
9.51E-6 |
year |
300.000 |
seconds ( x⁰ ) |
? |
% |
β+ |
4,102.000 |
keV |
¹⁹³Tl |
¹⁹³Pb > [ ? % , β+ , 4,102.0 keV ] > ¹⁹³Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁸⁹Os |
? |
% |
¹⁹³Ir |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_194_u |
Unstable |
¹⁹⁴Pb |
Boson |
82 |
p |
112 |
n |
0 |
1 |
193.974'012'070'0 |
u |
~ 0 |
% |
~ 0 |
-24.207'600'000'0 |
MeV |
7.865'416'000'0 |
MeV |
- |
|
- |
|
2.28E-5 |
year |
720.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
1,597.000 |
keV |
¹⁹⁴Tl |
¹⁹⁴Pb > [ 100 % , β+ , 1,597.0 keV ] > ¹⁹⁴Tl |
|
|
α |
4,737.900 |
keV |
¹⁹⁰Hg |
¹⁹⁴Pb > [ , α , 4,737.9 keV ] > ¹⁹⁰Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹⁴Pt |
? |
% |
¹⁹⁰Os |
? |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_195_u |
Unstable |
¹⁹⁵Pb |
Fermion |
82 |
p |
113 |
n |
3/2 |
-1 |
194.974'542'050'0 |
u |
~ 0 |
% |
~ 0 |
-23.713'927'000'0 |
MeV |
7.863'940'000'0 |
MeV |
- |
|
- |
|
2.85E-5 |
year |
900.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
3,418.900 |
keV |
¹⁹⁵Tl |
¹⁹⁵Pb > [ 100 % , β+ , 3,418.9 keV ] > ¹⁹⁵Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹⁵Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_196_u |
Unstable |
¹⁹⁶Pb |
Boson |
82 |
p |
114 |
n |
0 |
1 |
195.972'774'109'0 |
u |
~ 0 |
% |
~ 0 |
-25.360'754'000'0 |
MeV |
7.873'400'000'0 |
MeV |
- |
|
- |
|
6.97E-5 |
year |
2.200 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
1,113.700 |
keV |
¹⁹⁶Tl |
¹⁹⁶Pb > [ 100 % , β+ , 1,113.7 keV ] > ¹⁹⁶Tl |
|
|
α |
4,225.800 |
keV |
¹⁹²Hg |
¹⁹⁶Pb > [ , α , 4,225.8 keV ] > ¹⁹²Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.000'030 |
% |
¹⁹²Pt |
0.000'000 |
% |
¹⁸⁸Os |
? |
% |
¹⁹⁶Pt |
? |
% |
¹⁸⁰Hf |
? |
% |
¹⁷⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_197_u |
Unstable |
¹⁹⁷Pb |
Fermion |
82 |
p |
115 |
n |
3/2 |
-1 |
196.973'431'124'0 |
u |
~ 0 |
% |
~ 0 |
-24.748'749'000'0 |
MeV |
7.871'298'000'0 |
MeV |
-1.075'300'000'0 |
nm |
-0.080'000'000'0 |
b |
1.52E-5 |
year |
480.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
2,570.200 |
keV |
¹⁹⁷Tl |
¹⁹⁷Pb > [ 100 % , β+ , 2,570.2 keV ] > ¹⁹⁷Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹⁷Au |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_198_u |
Unstable |
¹⁹⁸Pb |
Boson |
82 |
p |
116 |
n |
0 |
1 |
197.972'033'959'0 |
u |
~ 0 |
% |
~ 0 |
-26.050'199'000'0 |
MeV |
7.878'882'000'0 |
MeV |
- |
|
- |
|
2.73E-4 |
year |
8.604 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
422.000 |
keV |
¹⁹⁸Tl |
¹⁹⁸Pb > [ 100 % , β+ , 422.0 keV ] > ¹⁹⁸Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹⁸Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_199_u |
Unstable |
¹⁹⁹Pb |
Fermion |
82 |
p |
117 |
n |
3/2 |
-1 |
198.972'916'650'0 |
u |
~ 0 |
% |
~ 0 |
-25.227'978'000'0 |
MeV |
7.875'717'000'0 |
MeV |
-1.074'200'000'0 |
nm |
0.080'000'000'0 |
b |
1.71E-4 |
year |
5.400 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
1,809.200 |
keV |
¹⁹⁹Tl |
¹⁹⁹Pb > [ 100 % , β+ , 1,809.2 keV ] > ¹⁹⁹Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹⁹Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_200_u |
Unstable |
²⁰⁰Pb |
Boson |
82 |
p |
118 |
n |
0 |
1 |
199.971'826'675'0 |
u |
~ 0 |
% |
~ 0 |
-26.243'283'000'0 |
MeV |
7.881'771'000'0 |
MeV |
- |
|
- |
|
2.45E-3 |
year |
77.397 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
ϵ |
804.800 |
keV |
²⁰⁰Tl |
²⁰⁰Pb > [ 100 % , ϵ , 804.8 keV ] > ²⁰⁰Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰⁰Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_201_u |
Unstable |
²⁰¹Pb |
Fermion |
82 |
p |
119 |
n |
5/2 |
-1 |
200.972'884'511'0 |
u |
~ 0 |
% |
~ 0 |
-25.257'915'000'0 |
MeV |
7.877'812'000'0 |
MeV |
0.675'300'000'0 |
nm |
-0.009'000'000'0 |
b |
1.06E-3 |
year |
33.599 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
901.900 |
keV |
²⁰¹Tl |
²⁰¹Pb > [ 100 % , β+ , 901.9 keV ] > ²⁰¹Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰¹Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_202_u |
Unstable |
²⁰²Pb |
Boson |
82 |
p |
120 |
n |
0 |
1 |
201.972'159'133'0 |
u |
~ 0 |
% |
~ 0 |
-25.933'600'000'0 |
MeV |
7.882'115'000'0 |
MeV |
- |
|
- |
|
5.26E+4 |
years |
1.661 |
tera-seconds ( x¹² ) |
100.000'000 |
% |
ϵ |
49.700 |
keV |
²⁰²Tl |
²⁰²Pb > [ 100 % , ϵ , 49.7 keV ] > ²⁰²Tl |
|
|
α |
2,595.930 |
keV |
¹⁹⁸Hg |
²⁰²Pb > [ , α , 2,595.93 keV ] > ¹⁹⁸Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰²Hg |
1.000'000 |
% |
¹⁹⁸Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_203_u |
Unstable |
²⁰³Pb |
Fermion |
82 |
p |
121 |
n |
5/2 |
-1 |
202.973'390'521'0 |
u |
~ 0 |
% |
~ 0 |
-24.786'570'000'0 |
MeV |
7.877'397'000'0 |
MeV |
0.686'400'000'0 |
nm |
0.095'000'000'0 |
b |
5.92E-3 |
year |
186.740 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
ϵ |
974.620 |
keV |
²⁰⁴Tl |
²⁰³Pb > [ 100 % , ϵ , 974.62 keV ] > ²⁰⁴Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰³Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_204_s |
Stable |
²⁰⁴Pb |
Boson |
82 |
p |
122 |
n |
0 |
1 |
203.973'043'589'0 |
u |
1.400'000 |
% |
2.855'622'610'2 |
-25.109'735'000'0 |
MeV |
7.879'931'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
? |
% |
α |
1,969.490 |
keV |
²⁰⁰Hg |
²⁰⁴Pb > [ ? % , α , 1,969.49 keV ] > ²⁰⁰Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
²⁰⁰Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_205_u |
Unstable |
²⁰⁵Pb |
Fermion |
82 |
p |
123 |
n |
5/2 |
-1 |
204.974'481'755'0 |
u |
~ 0 |
% |
~ 0 |
-23.770'091'000'0 |
MeV |
7.874'330'000'0 |
MeV |
0.711'700'000'0 |
nm |
0.226'000'000'0 |
b |
1.52E+7 |
years |
480.685 |
tera-seconds ( x¹² ) |
100.000'000 |
% |
ϵ |
50.500 |
keV |
²⁰⁵Tl |
²⁰⁵Pb > [ 100 % , ϵ , 50.5 keV ] > ²⁰⁵Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰⁵Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_206_s |
Stable |
²⁰⁶Pb |
Boson |
82 |
p |
124 |
n |
0 |
1 |
205.974'465'278'0 |
u |
24.100'000 |
% |
49.639'846'132'0 |
-23.785'440'000'0 |
MeV |
7.875'361'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_207_s |
Stable |
²⁰⁷Pb |
Fermion |
82 |
p |
125 |
n |
1/2 |
-1 |
206.975'896'887'0 |
u |
22.100'000 |
% |
45.741'673'212'0 |
-22.451'905'000'0 |
MeV |
7.869'865'000'0 |
MeV |
0.592'583'000'0 |
nm |
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_208_s |
Stable |
²⁰⁸Pb |
Boson |
82 |
p |
126 |
n |
0 |
1 |
207.976'652'071'0 |
u |
52.400'000 |
% |
108.979'765'685'2 |
-21.748'455'000'0 |
MeV |
7.867'452'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_209_u |
Unstable |
²⁰⁹Pb |
Fermion |
82 |
p |
127 |
n |
9/2 |
1 |
208.981'090'120'0 |
u |
~ 0 |
% |
~ 0 |
-17.614'440'000'0 |
MeV |
7.848'647'000'0 |
MeV |
-1.473'500'000'0 |
nm |
-0.270'000'000'0 |
b |
3.71E-4 |
year |
11.710 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
644.020 |
keV |
²⁰⁹Bi |
²⁰⁹Pb > [ 100 % , β- , 644.02 keV ] > ²⁰⁹Bi |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰⁵Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_210_u |
Unstable |
²¹⁰Pb |
Boson |
82 |
p |
128 |
n |
0 |
1 |
209.984'188'527'0 |
u |
~ 0 |
% |
~ 0 |
-14.728'292'000'0 |
MeV |
7.835'964'000'0 |
MeV |
- |
|
- |
|
2.22E+1 |
years |
701.525 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
β- |
63.486 |
keV |
²¹⁰Bi |
²¹⁰Pb > [ 100 % , β- , 63.486 keV ] > ²¹⁰Bi |
|
|
α |
3,792.300 |
keV |
²⁰⁶Hg |
²¹⁰Pb > [ , α , 3,792.3 keV ] > ²⁰⁶Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'134 |
% |
²⁰⁶Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_211_u |
Unstable |
²¹¹Pb |
Fermion |
82 |
p |
129 |
n |
9/2 |
1 |
210.988'736'964'0 |
u |
~ 0 |
% |
~ 0 |
-10.491'450'000'0 |
MeV |
7.817'000'000'0 |
MeV |
-1.403'700'000'0 |
nm |
0.087'000'000'0 |
b |
6.88E-5 |
year |
2.170 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
1,366.970 |
keV |
²¹¹Bi |
²¹¹Pb > [ 100 % , β- , 1,366.97 keV ] > ²¹¹Bi |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.276'000 |
% |
²⁰⁷Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_212_u |
Unstable |
²¹²Pb |
Boson |
82 |
p |
130 |
n |
0 |
1 |
211.991'897'543'0 |
u |
~ 0 |
% |
~ 0 |
-7.547'389'000'0 |
MeV |
7.804'312'000'0 |
MeV |
- |
|
- |
|
1.21E-3 |
year |
38.304 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
569.910 |
keV |
²¹²Bi |
²¹²Pb > [ 100 % , β- , 569.91 keV ] > ²¹²Bi |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.004'000 |
% |
²⁰⁸Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_213_u |
Unstable |
²¹³Pb |
Fermion |
82 |
p |
131 |
n |
9/2 |
1 |
212.996'581'499'0 |
u |
~ 0 |
% |
~ 0 |
-3.184'313'000'0 |
MeV |
7.785'082'000'0 |
MeV |
- |
|
- |
|
1.94E-5 |
year |
612.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
2,046.340 |
keV |
²¹³Bi |
²¹³Pb > [ 100 % , β- , 2,046.34 keV ] > ²¹³Bi |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰⁵Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_214_u |
Unstable |
²¹⁴Pb |
Boson |
82 |
p |
132 |
n |
0 |
1 |
213.999'805'408'0 |
u |
~ 0 |
% |
~ 0 |
-0.181'261'000'0 |
MeV |
7.772'386'000'0 |
MeV |
- |
|
- |
|
5.10E-5 |
year |
1.610 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
1,018.900 |
keV |
²¹⁴Bi |
²¹⁴Pb > [ 100 % , β- , 1,018.9 keV ] > ²¹⁴Bi |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.024'134 |
% |
²⁰⁶Pb |
0.000'002 |
% |
²⁰⁵Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_082_pb_215_u |
Unstable |
²¹⁵Pb |
Fermion |
82 |
p |
133 |
n |
5/2 |
1 |
215.004'807'000'0 |
u |
~ 0 |
% |
~ 0 |
4.477'000'000'0 |
MeV |
7.752'000'000'0 |
MeV |
- |
|
- |
|
1.14E-6 |
year |
36.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
2,829.000 |
keV |
²¹⁵Bi |
²¹⁵Pb > [ 100 % , β- , 2,829.0 keV ] > ²¹⁵Bi |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.276'231 |
% |
²⁰⁷Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|