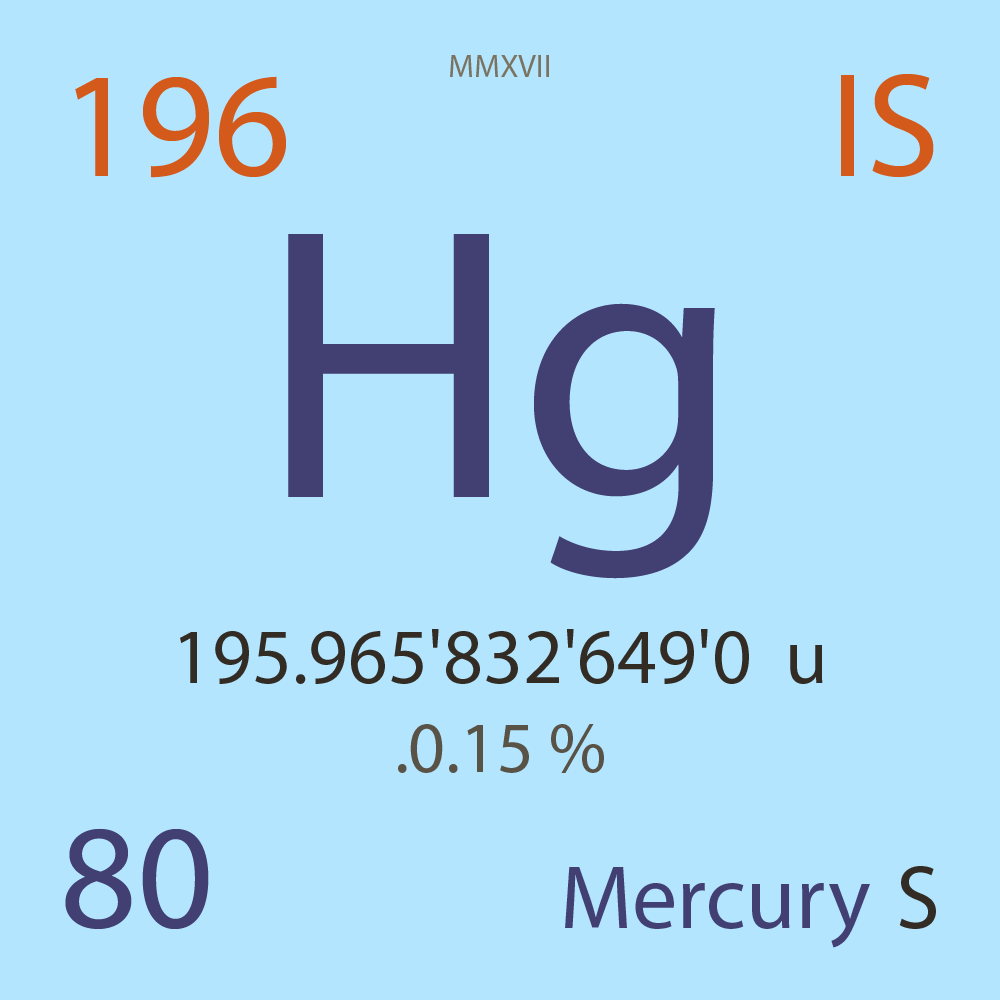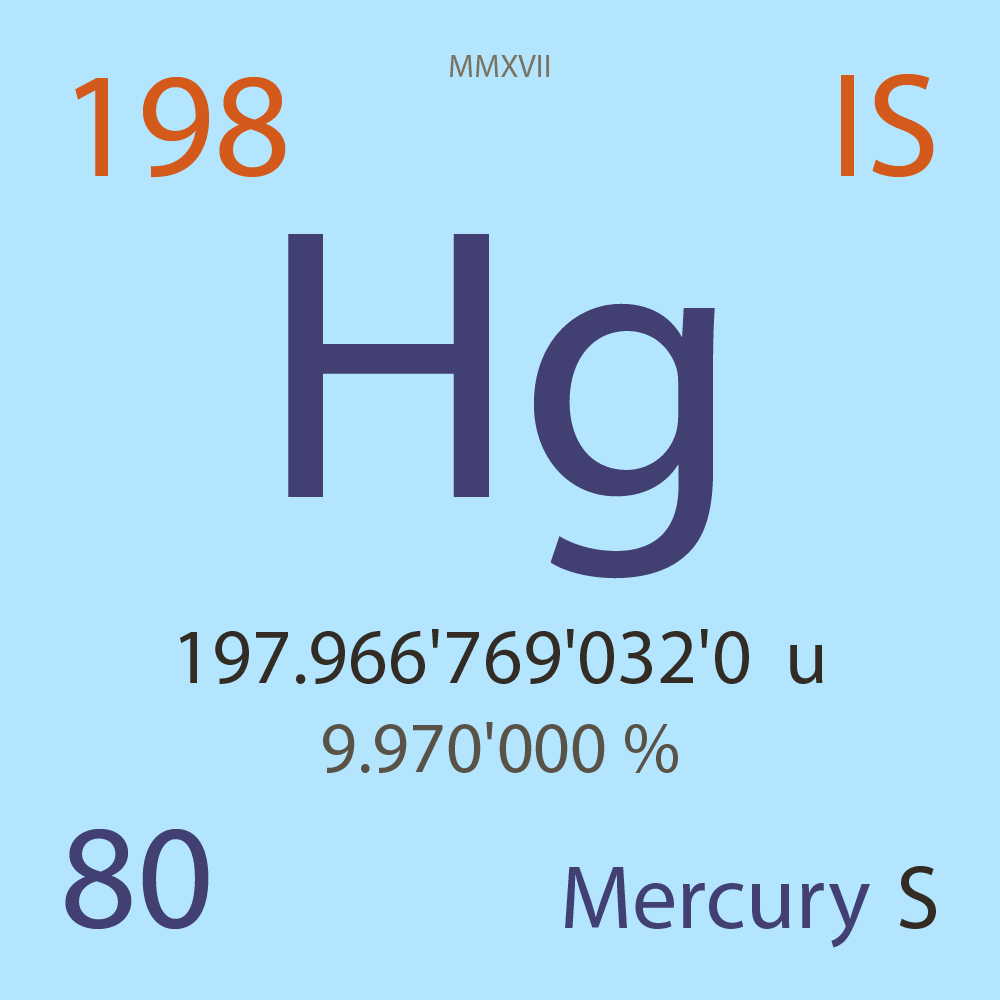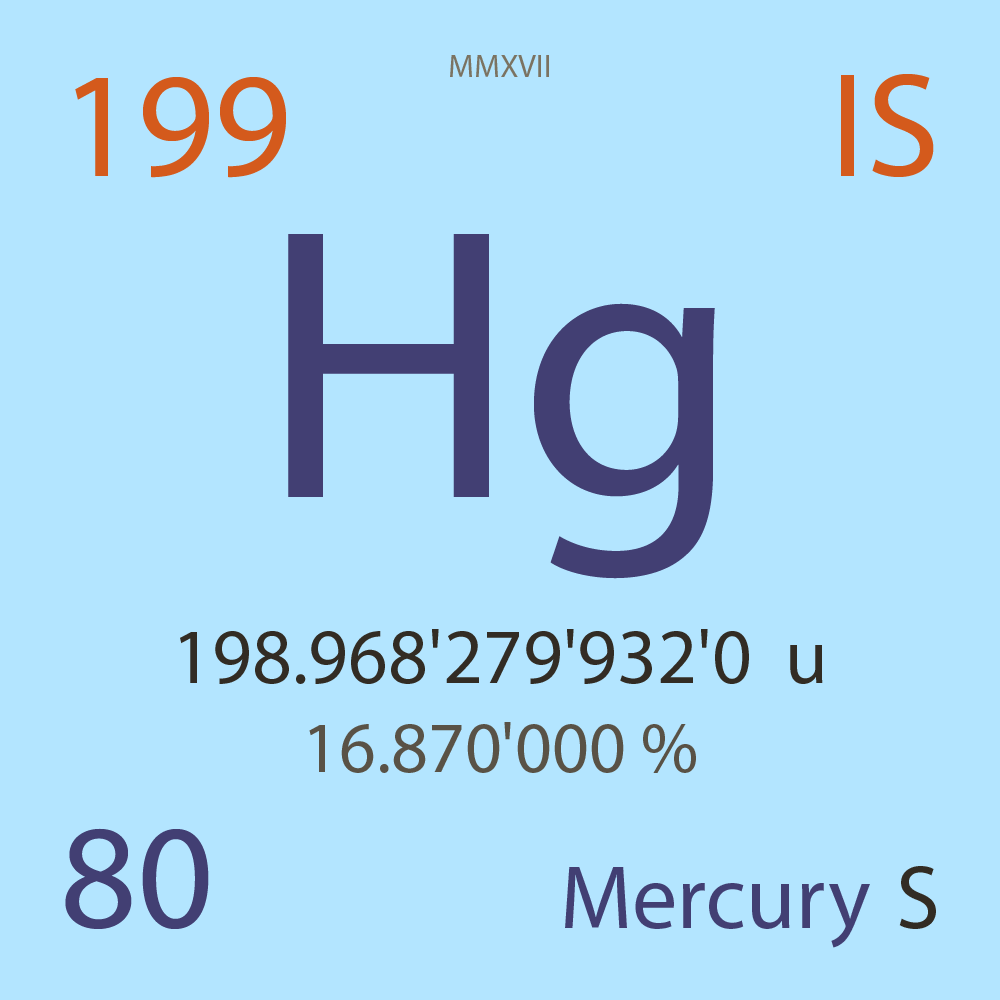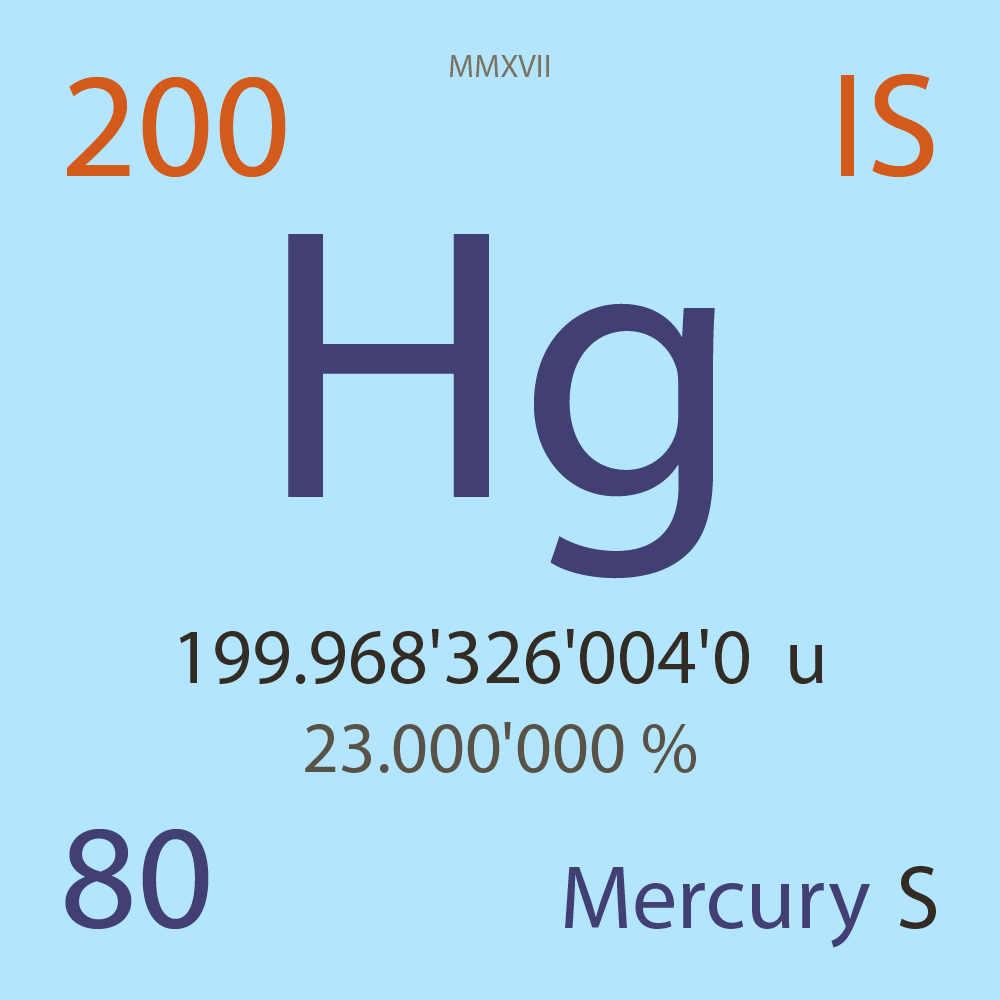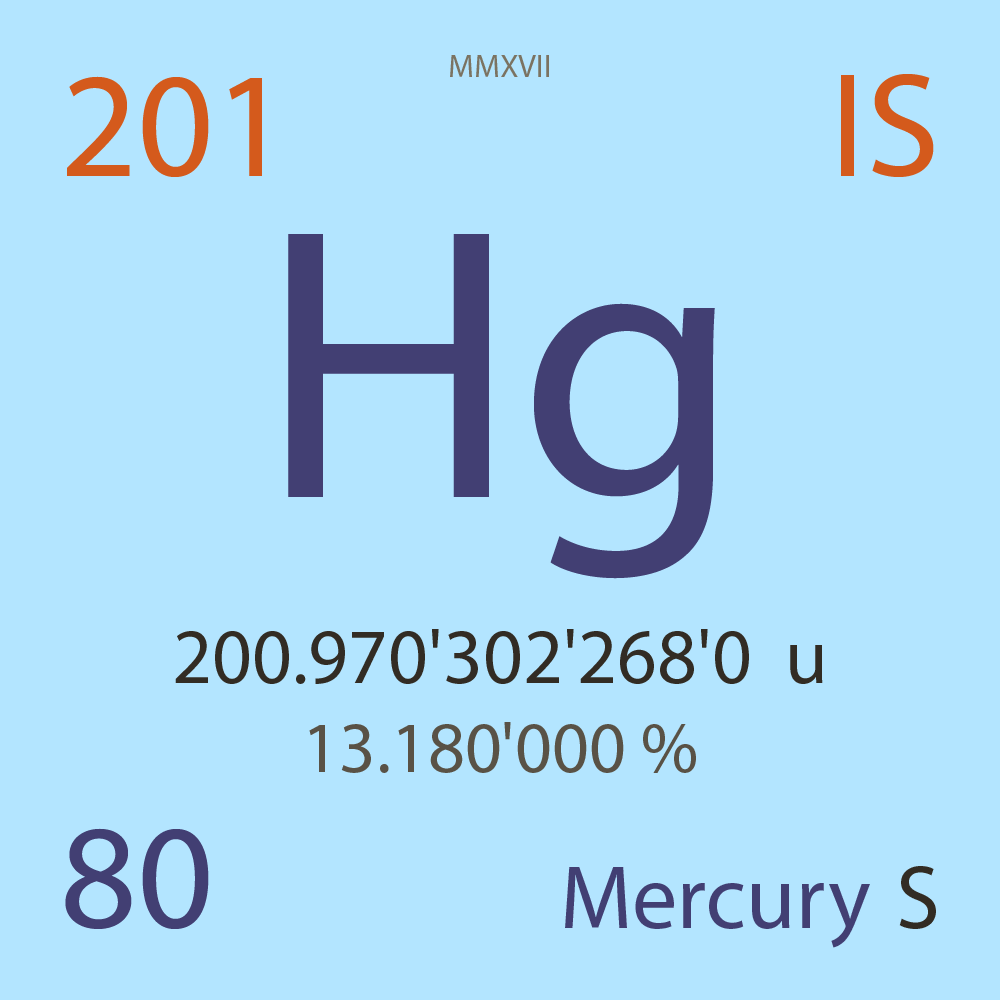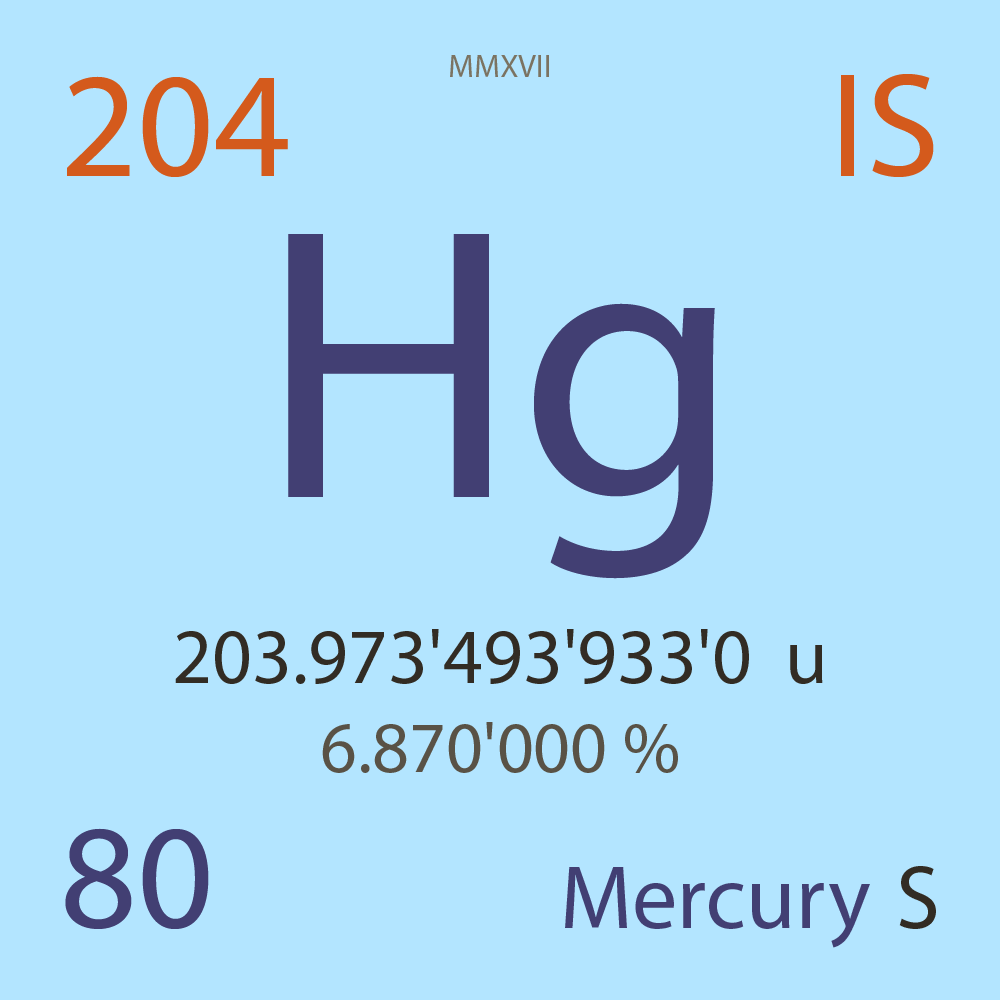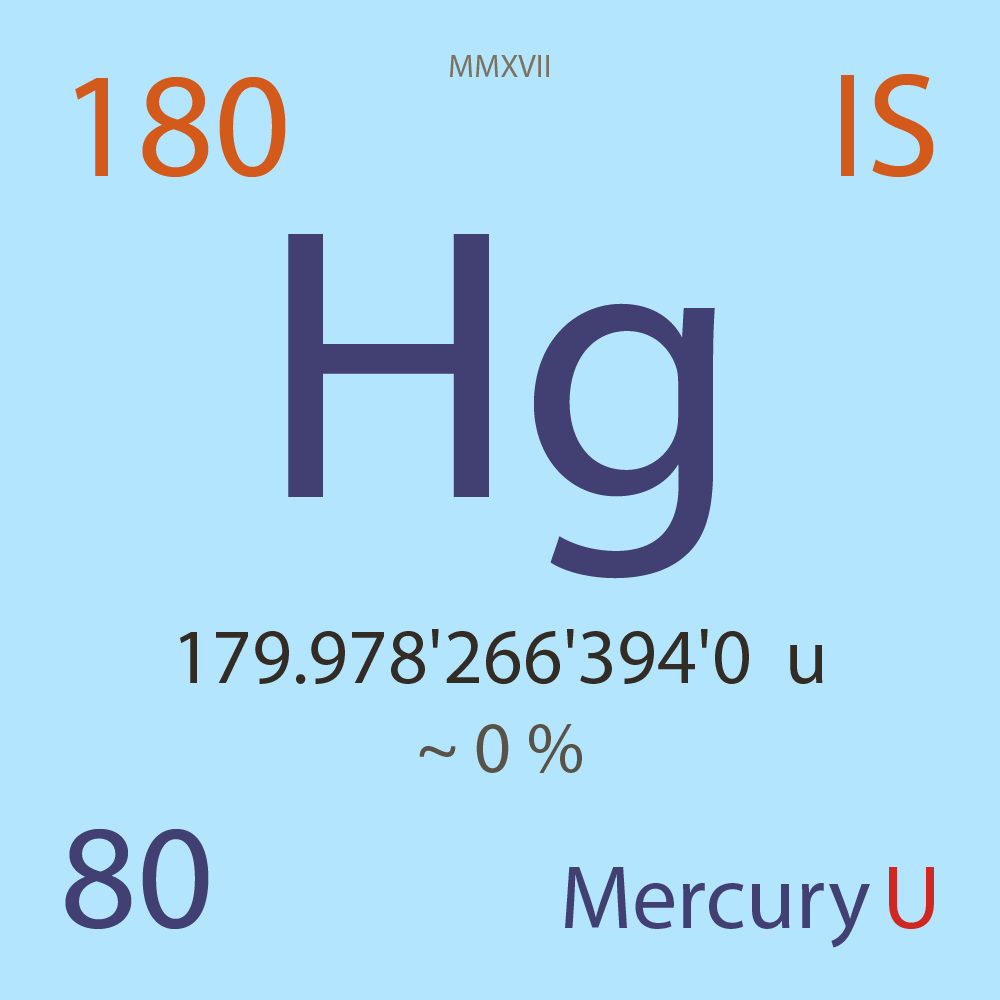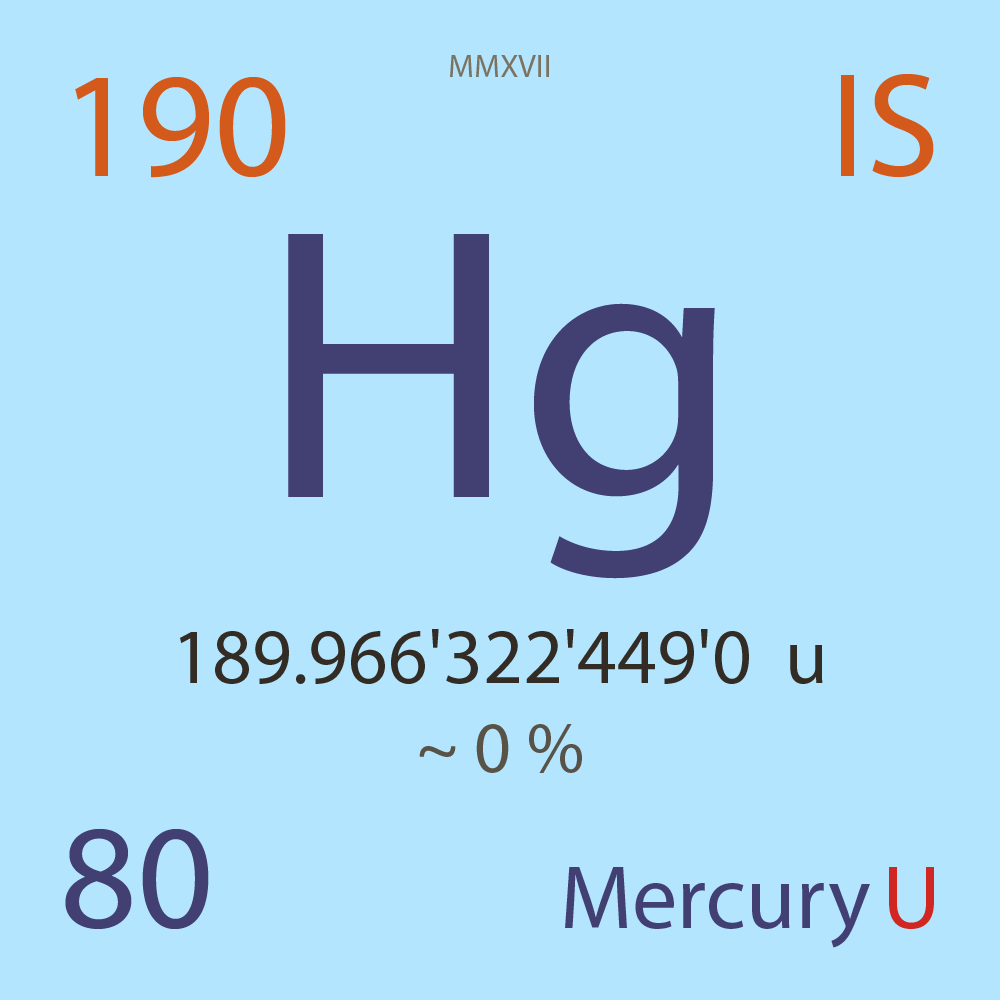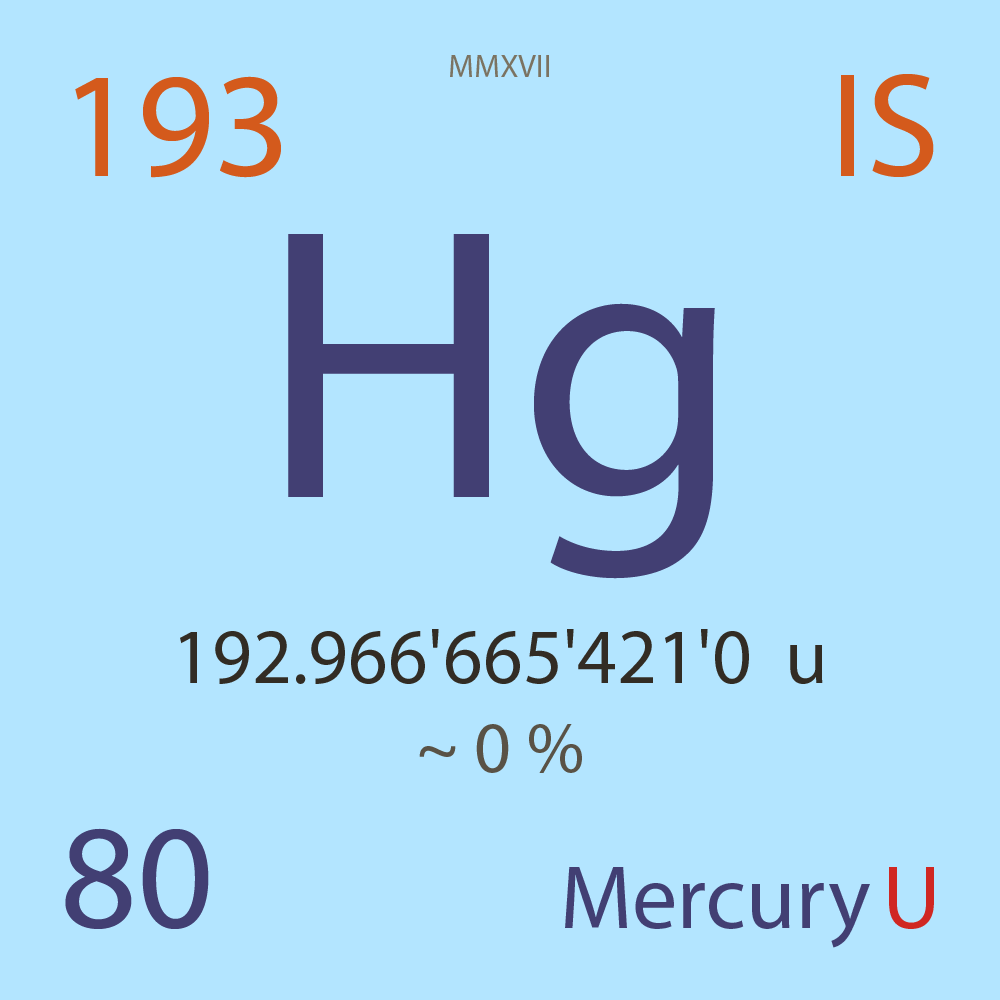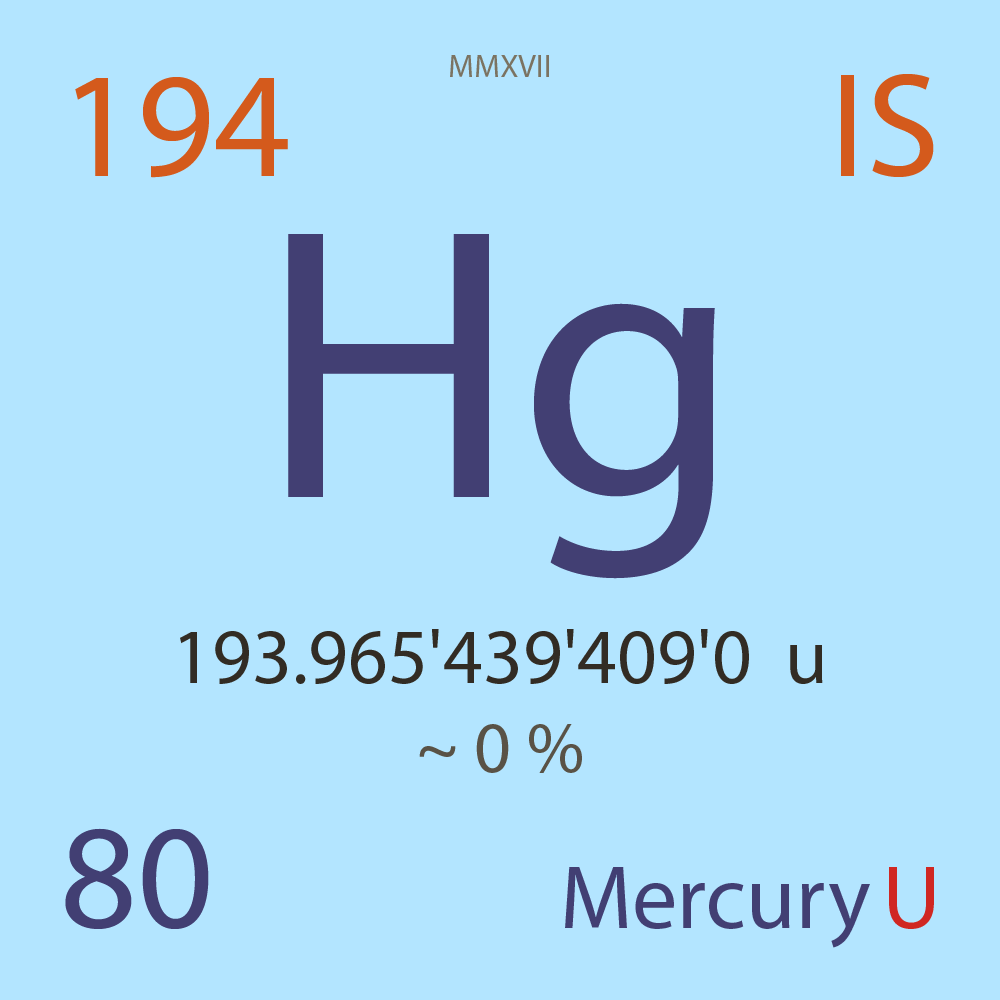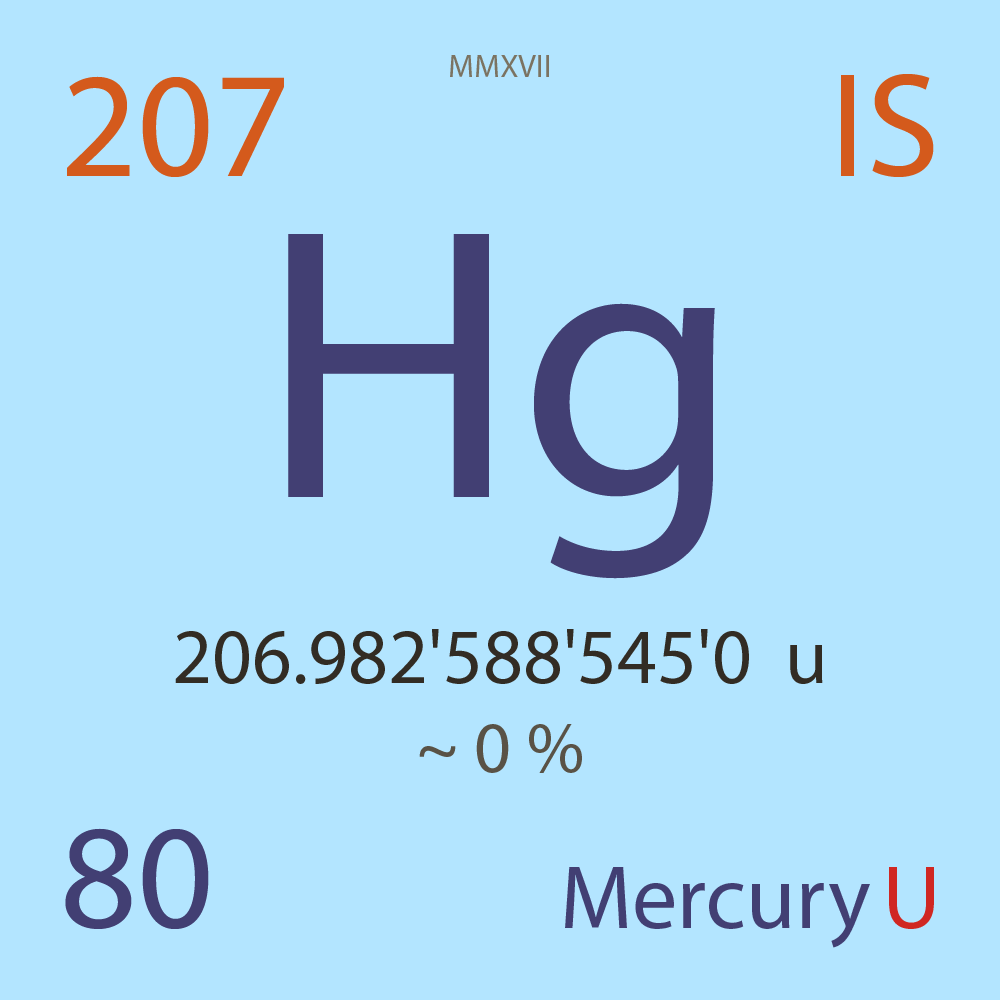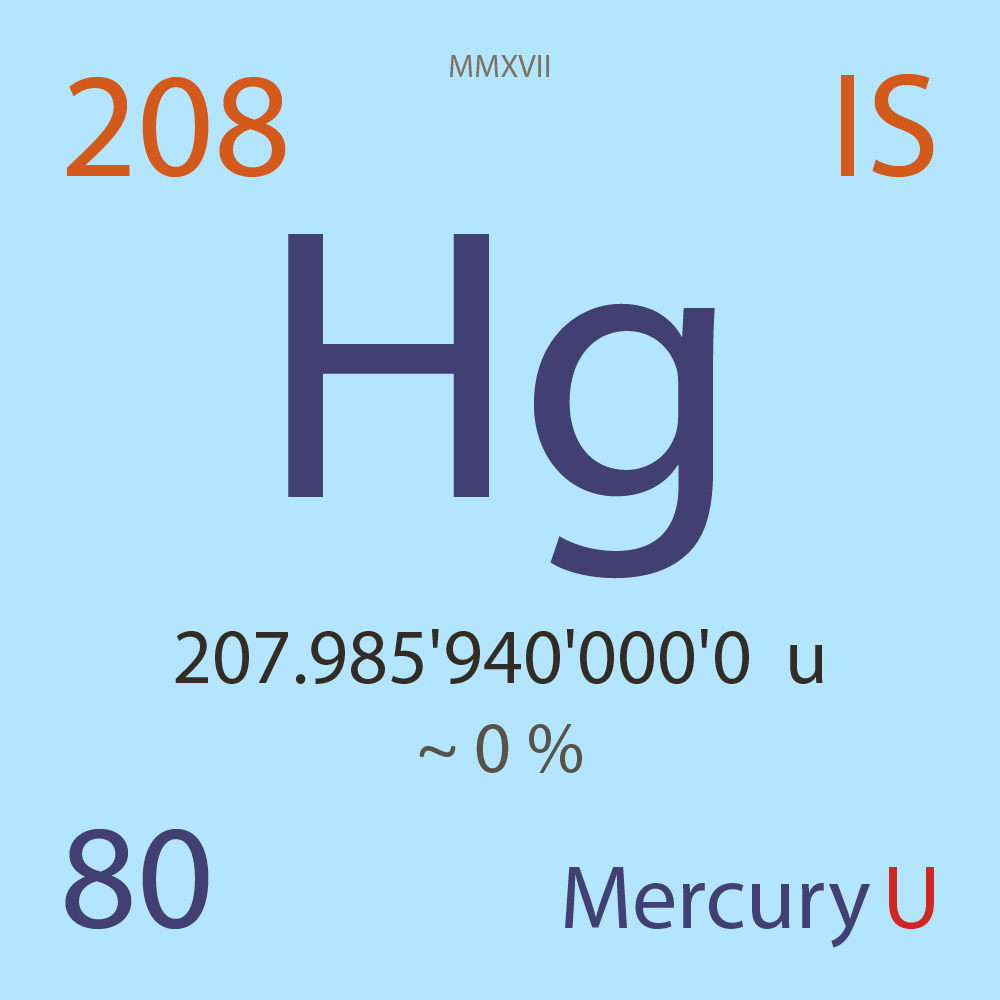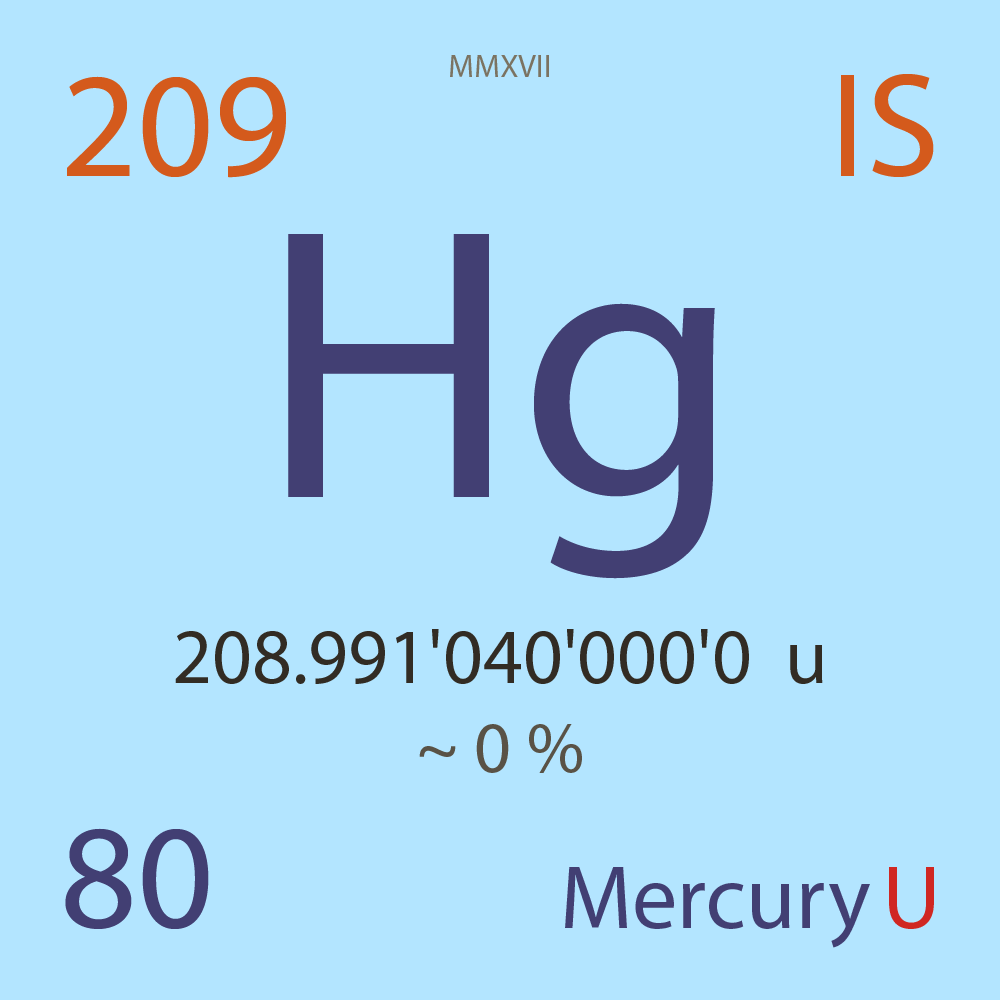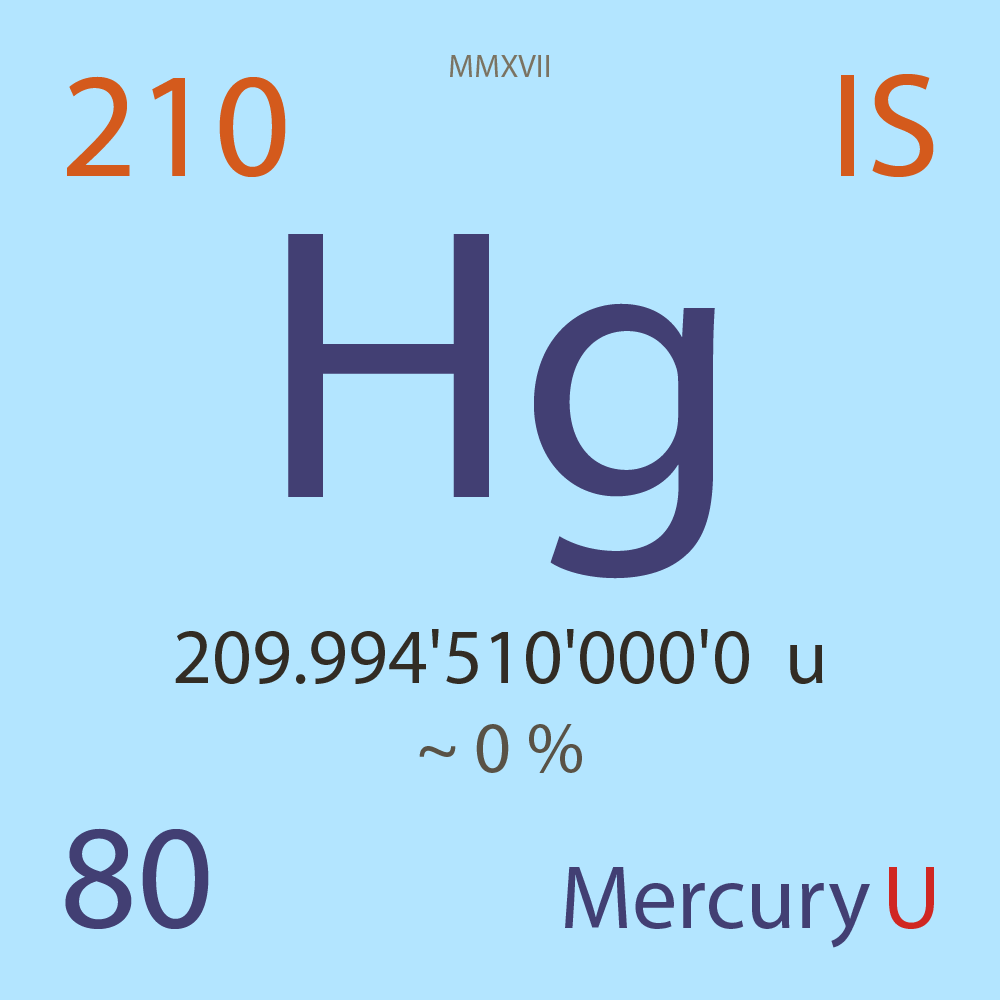| Isotope_080_hg_171_u |
Unstable |
¹⁷¹Hg |
Fermion |
80 |
p |
91 |
n |
3/2 |
-1 |
171.003'760'000'0 |
u |
~ 0 |
% |
~ 0 |
3.502'000'000'0 |
MeV |
7.685'000'000'0 |
MeV |
- |
|
- |
|
2.54E-12 |
year |
80.000 |
micro-seconds ( x⁻⁶ ) |
100.000'000 |
% |
α |
7,617.000 |
keV |
¹⁶⁷Pt |
¹⁷¹Hg > [ 100 % , α , 7,617.0 keV ] > ¹⁶⁷Pt |
|
|
β+ |
10,045.000 |
keV |
¹⁷¹Au |
¹⁷¹Hg > [ , β+ , 10,045.0 keV ] > ¹⁷¹Au |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
53.132'851 |
% |
¹⁵¹Eu |
19.032'616 |
% |
¹⁴³Nd |
0.001'452 |
% |
¹⁴³Nd |
0.000'173 |
% |
¹⁷⁰Yb |
0.000'018 |
% |
¹⁶⁶Er |
? |
% |
¹⁵⁹Tb |
? |
% |
¹⁵⁵Gd |
? |
% |
¹⁵⁴Gd |
? |
% |
¹⁶⁷Er |
? |
% |
¹⁶³Dy |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁸Gd |
| Isotope_080_hg_172_u |
Unstable |
¹⁷²Hg |
Boson |
80 |
p |
92 |
n |
0 |
1 |
171.998'832'686'0 |
u |
~ 0 |
% |
~ 0 |
-1.087'345'000'0 |
MeV |
7.713'757'000'0 |
MeV |
- |
|
- |
|
1.33E-11 |
year |
420.000 |
micro-seconds ( x⁻⁶ ) |
100.000'000 |
% |
α |
7,525.300 |
keV |
¹⁶⁸Pt |
¹⁷²Hg > [ 100 % , α , 7,525.3 keV ] > ¹⁶⁸Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7.277'794 |
% |
¹⁴⁰Ce |
? |
% |
¹⁴³Nd |
? |
% |
¹⁵¹Eu |
? |
% |
¹⁶⁰Dy |
? |
% |
¹⁵²Sm |
? |
% |
¹⁵⁶Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_173_u |
Unstable |
¹⁷³Hg |
Fermion |
80 |
p |
93 |
n |
3/2 |
-1 |
172.997'242'000'0 |
u |
~ 0 |
% |
~ 0 |
-2.569'000'000'0 |
MeV |
7.724'7.724'7.724'7.724 |
MeV |
- |
|
- |
|
3.49E-11 |
year |
1.100 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
α |
7,381.000 |
keV |
¹⁶⁹Pt |
¹⁷³Hg > [ 100 % , α , 7,381.0 keV ] > ¹⁶⁹Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.895'920 |
% |
¹⁴⁵Nd |
0.000'263 |
% |
¹⁴⁰Ce |
0.000'000 |
% |
¹⁴¹Pr |
? |
% |
¹⁶⁹Tm |
? |
% |
¹⁶⁵Ho |
? |
% |
¹⁵⁷Gd |
? |
% |
¹⁵³Eu |
? |
% |
¹⁶¹Dy |
? |
% |
¹⁵²Sm |
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_174_u |
Unstable |
¹⁷⁴Hg |
Boson |
80 |
p |
94 |
n |
0 |
1 |
173.992'863'695'0 |
u |
~ 0 |
% |
~ 0 |
-6.647'425'000'0 |
MeV |
7.749'821'000'0 |
MeV |
- |
|
- |
|
6.34E-11 |
year |
2.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
α |
7,233.190 |
keV |
¹⁷⁰Pt |
¹⁷⁴Hg > [ 100 % , α , 7,233.19 keV ] > ¹⁷⁰Pt |
|
|
β+ |
6,526.000 |
keV |
¹⁷⁴Au |
¹⁷⁴Hg > [ , β+ , 6,526.0 keV ] > ¹⁷⁴Au |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
14.519'370 |
% |
¹⁴²Nd |
2.044'874 |
% |
¹⁷⁰Yb |
0.002'131 |
% |
¹⁶⁶Er |
? |
% |
¹⁵⁴Gd |
? |
% |
¹⁷⁴Yb |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁸Gd |
? |
% |
¹⁶²Dy |
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_175_u |
Unstable |
¹⁷⁵Hg |
Fermion |
80 |
p |
95 |
n |
5/2 |
-1 |
174.991'423'270'0 |
u |
~ 0 |
% |
~ 0 |
-7.989'172'000'0 |
MeV |
7.759'325'000'0 |
MeV |
- |
|
- |
|
3.42E-10 |
year |
10.800 |
milli-seconds ( x⁻³ ) |
99.000'000 |
% |
α |
7,056.500 |
keV |
¹⁷¹Pt |
¹⁷⁵Hg > [ 99 % , α , 7,056.5 keV ] > ¹⁷¹Pt |
|
|
β+ |
8,432.000 |
keV |
¹⁷⁵Au |
¹⁷⁵Hg > [ , β+ , 8,432.0 keV ] > ¹⁷⁵Au |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
91.810'818 |
% |
¹⁶³Dy |
4.856'682 |
% |
¹⁵⁹Tb |
1.652'935 |
% |
¹⁵¹Eu |
0.592'077 |
% |
¹⁴³Nd |
0.276'289 |
% |
¹⁵⁵Gd |
? |
% |
¹⁷¹Yb |
? |
% |
¹⁷⁵Lu |
? |
% |
¹⁶⁷Er |
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_176_u |
Unstable |
¹⁷⁶Hg |
Boson |
80 |
p |
96 |
n |
0 |
1 |
175.987'354'580'0 |
u |
~ 0 |
% |
~ 0 |
-11.779'132'000'0 |
MeV |
7.782'632'000'0 |
MeV |
- |
|
- |
|
6.46E-10 |
year |
20.400 |
milli-seconds ( x⁻³ ) |
90.000'000 |
% |
α |
6,896.970 |
keV |
¹⁷²Pt |
¹⁷⁶Hg > [ 90 % , α , 6,896.97 keV ] > ¹⁷²Pt |
|
|
β+ |
5,736.000 |
keV |
¹⁷⁶Au |
¹⁷⁶Hg > [ , β+ , 5,736.0 keV ] > ¹⁷⁶Au |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.281'333 |
% |
¹⁶⁰Dy |
0.000'084 |
% |
¹⁴⁰Ce |
? |
% |
¹⁷²Yb |
? |
% |
¹⁷⁶Hf |
? |
% |
¹⁵²Sm |
? |
% |
¹⁵⁸Gd |
? |
% |
¹⁶⁸Er |
? |
% |
¹⁶⁴Dy |
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_177_u |
Unstable |
¹⁷⁷Hg |
Fermion |
80 |
p |
97 |
n |
5/2 |
-1 |
176.986'279'158'0 |
u |
~ 0 |
% |
~ 0 |
-12.780'882'000'0 |
MeV |
7.789'922'000'0 |
MeV |
- |
|
- |
|
4.03E-9 |
year |
127.300 |
milli-seconds ( x⁻³ ) |
85.000'000 |
% |
α |
6,735.800 |
keV |
¹⁷³Pt |
¹⁷⁷Hg > [ 85 % , α , 6,735.8 keV ] > ¹⁷³Pt |
|
|
β+ |
7,747.100 |
keV |
¹⁷⁷Au |
¹⁷⁷Hg > [ , β+ , 7,747.1 keV ] > ¹⁷⁷Au |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
65.651'115 |
% |
¹⁶⁹Tm |
26.598'000 |
% |
¹⁷³Yb |
7.857'277 |
% |
¹⁶⁵Ho |
0.015'708 |
% |
¹⁶¹Dy |
0.000'020 |
% |
¹⁵⁷Gd |
0.000'000 |
% |
¹⁵³Eu |
0.000'000 |
% |
¹⁴⁵Nd |
0.000'000 |
% |
¹⁴¹Pr |
? |
% |
¹⁷⁷Hf |
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_178_u |
Unstable |
¹⁷⁸Hg |
Boson |
80 |
p |
98 |
n |
0 |
1 |
177.982'483'143'0 |
u |
~ 0 |
% |
~ 0 |
-16.316'846'000'0 |
MeV |
7.811'368'000'0 |
MeV |
- |
|
- |
|
8.52E-9 |
year |
269.000 |
milli-seconds ( x⁻³ ) |
70.000'000 |
% |
α |
6,577.390 |
keV |
¹⁷⁴Pt |
¹⁷⁸Hg > [ 70 % , α , 6,577.39 keV ] > ¹⁷⁴Pt |
|
|
β+ |
4,987.100 |
keV |
¹⁷⁸Au |
¹⁷⁸Hg > [ , β+ , 4,987.1 keV ] > ¹⁷⁸Au |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
62.013'998 |
% |
¹⁷⁰Yb |
16.614'000 |
% |
¹⁷⁸Hf |
5.066'948 |
% |
¹⁶⁶Er |
0.000'000 |
% |
¹⁴²Nd |
? |
% |
¹⁵⁴Gd |
? |
% |
¹⁷⁴Yb |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁸Gd |
? |
% |
¹⁶²Dy |
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_179_u |
Unstable |
¹⁷⁹Hg |
Fermion |
80 |
p |
99 |
n |
5/2 |
-1 |
178.981'833'861'0 |
u |
~ 0 |
% |
~ 0 |
-16.921'649'000'0 |
MeV |
7.816'199'000'0 |
MeV |
- |
|
- |
|
3.45E-8 |
year |
1.090 |
seconds ( x⁰ ) |
53.000'000 |
% |
α |
6,343.500 |
keV |
¹⁷⁵Pt |
¹⁷⁹Hg > [ 53 % , α , 6,343.5 keV ] > ¹⁷⁵Pt |
|
|
β+ |
? |
keV |
¹⁷⁸Pt |
¹⁷⁹Hg > [ , β+ , ? keV ] > ¹⁷⁸Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
33.309'440 |
% |
¹⁷¹Yb |
0.610'316 |
% |
¹⁶⁷Er |
0.138'450 |
% |
¹⁷⁸Hf |
0.011'553 |
% |
¹⁷⁰Yb |
0.000'244 |
% |
¹⁶³Dy |
0.000'000 |
% |
¹⁶⁶Er |
0.000'000 |
% |
¹⁵⁹Tb |
? |
% |
¹⁷⁵Lu |
? |
% |
¹⁷⁴Yb |
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_180_u |
Unstable |
¹⁸⁰Hg |
Boson |
80 |
p |
100 |
n |
0 |
1 |
179.978'266'394'0 |
u |
~ 0 |
% |
~ 0 |
-20.244'723'000'0 |
MeV |
7.836'078'000'0 |
MeV |
- |
|
- |
|
8.11E-8 |
year |
2.560 |
seconds ( x⁰ ) |
52.000'000 |
% |
β+ |
4,329.500 |
keV |
¹⁸⁰Au |
¹⁸⁰Hg > [ 52 % , β+ , 4,329.5 keV ] > ¹⁸⁰Au |
|
|
α |
6,258.260 |
keV |
¹⁷⁵Pt |
¹⁸⁰Hg > [ , α , 6,258.26 keV ] > ¹⁷⁵Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
29.897'616 |
% |
¹⁷⁶Hf |
18.990'936 |
% |
¹⁷²Yb |
? |
% |
¹⁸⁰Hf |
? |
% |
¹⁶⁸Er |
? |
% |
¹⁶⁴Dy |
? |
% |
¹⁶⁰Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_181_u |
Unstable |
¹⁸¹Hg |
Fermion |
80 |
p |
101 |
n |
1/2 |
-1 |
180.977'819'311'0 |
u |
~ 0 |
% |
~ 0 |
-20.661'178'000'0 |
MeV |
7.839'679'000'0 |
MeV |
0.507'100'000'0 |
nm |
- |
|
1.14E-7 |
year |
3.600 |
seconds ( x⁰ ) |
31.000'000 |
% |
α |
6,284.390 |
keV |
¹⁷⁷Pt |
¹⁸¹Hg > [ 31 % , α , 6,284.39 keV ] > ¹⁷⁷Pt |
|
|
β+p |
? |
keV |
¹⁸⁰Pt |
¹⁸¹Hg > [ , β+p , ? keV ] > ¹⁸⁰Pt |
0.000'011 |
% |
β+α |
? |
keV |
¹⁷⁷Ir |
¹⁸¹Hg > [ 0.000011 % , β+α , ? keV ] > ¹⁷⁷Ir |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
29.233'011 |
% |
¹⁷⁷Hf |
1.784'540 |
% |
¹⁷³Yb |
0.007'068 |
% |
¹⁶⁹Tm |
0.000'048 |
% |
¹⁷⁶Hf |
? |
% |
¹⁸⁰Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_182_u |
Unstable |
¹⁸²Hg |
Boson |
80 |
p |
102 |
n |
0 |
1 |
181.974'689'964'0 |
u |
~ 0 |
% |
~ 0 |
-23.576'146'000'0 |
MeV |
7.856'968'000'0 |
MeV |
- |
|
- |
|
3.43E-7 |
year |
10.830 |
seconds ( x⁰ ) |
86.200'000 |
% |
β+ |
3,702.400 |
keV |
¹⁸²Au |
¹⁸²Hg > [ 86.2 % , β+ , 3,702.4 keV ] > ¹⁸²Au |
|
|
α |
5,996.950 |
keV |
¹⁷⁸Pt |
¹⁸²Hg > [ , α , 5,996.95 keV ] > ¹⁷⁸Pt |
0.000'010 |
% |
β+p |
? |
keV |
¹⁸¹Pt |
¹⁸²Hg > [ 0.00001 % , β+p , ? keV ] > ¹⁸¹Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12.882'216 |
% |
¹⁷⁸Hf |
1.062'855 |
% |
¹⁷⁰Yb |
0.000'010 |
% |
¹⁸¹Ta |
0.000'003 |
% |
¹⁶⁶Er |
0.000'000 |
% |
¹⁷⁷Hf |
? |
% |
¹⁷⁴Yb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_183_u |
Unstable |
¹⁸³Hg |
Fermion |
80 |
p |
103 |
n |
1/2 |
-1 |
182.974'449'841'0 |
u |
~ 0 |
% |
~ 0 |
-23.799'819'000'0 |
MeV |
7.859'361'000'0 |
MeV |
0.524'000'000'0 |
nm |
- |
|
2.98E-7 |
year |
9.400 |
seconds ( x⁰ ) |
88.300'000 |
% |
β+ |
5,364.900 |
keV |
¹⁸³Au |
¹⁸³Hg > [ 88.3 % , β+ , 5,364.9 keV ] > ¹⁸³Au |
|
|
α |
6,039.050 |
keV |
¹⁷⁹Pt |
¹⁸³Hg > [ , α , 6,039.05 keV ] > ¹⁷⁹Pt |
0.000'260 |
% |
β+p |
? |
keV |
¹⁸²Pt |
¹⁸³Hg > [ 0.00026 % , β+p , ? keV ] > ¹⁸²Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12.238'277 |
% |
¹⁷⁹Hf |
0.028'080 |
% |
¹⁷⁵Lu |
0.000'000 |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_184_u |
Unstable |
¹⁸⁴Hg |
Boson |
80 |
p |
104 |
n |
0 |
1 |
183.971'713'051'0 |
u |
~ 0 |
% |
~ 0 |
-26.349'123'000'0 |
MeV |
7.874'368'000'0 |
MeV |
- |
|
- |
|
9.70E-7 |
year |
30.600 |
seconds ( x⁰ ) |
98.890'000 |
% |
β+ |
2,947.400 |
keV |
¹⁸⁴Au |
¹⁸⁴Hg > [ 98.89 % , β+ , 2,947.4 keV ] > ¹⁸⁴Au |
|
|
α |
5,661.920 |
keV |
¹⁸⁰Pt |
¹⁸⁴Hg > [ , α , 5,661.92 keV ] > ¹⁸⁰Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.003'330 |
% |
¹⁷⁶Hf |
? |
% |
¹⁸⁰Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_185_u |
Unstable |
¹⁸⁵Hg |
Fermion |
80 |
p |
105 |
n |
1/2 |
-1 |
184.971'899'086'0 |
u |
~ 0 |
% |
~ 0 |
-26.175'833'000'0 |
MeV |
7.874'496'000'0 |
MeV |
0.508'000'000'0 |
nm |
- |
|
1.56E-6 |
year |
49.100 |
seconds ( x⁰ ) |
94.000'000 |
% |
β+ |
4,668.900 |
keV |
¹⁸⁵Au |
¹⁸⁵Hg > [ 94 % , β+ , 4,668.9 keV ] > ¹⁸⁵Au |
|
|
α |
5,773.910 |
keV |
¹⁸¹Pt |
¹⁸⁵Hg > [ , α , 5,773.91 keV ] > ¹⁸¹Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
94.000'000 |
% |
¹⁸⁵Re |
6.249'100 |
% |
¹⁸¹Ta |
0.004'440 |
% |
¹⁷⁷Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_186_u |
Unstable |
¹⁸⁶Hg |
Boson |
80 |
p |
106 |
n |
0 |
1 |
185.969'361'790'0 |
u |
~ 0 |
% |
~ 0 |
-28.539'309'000'0 |
MeV |
7.888'261'000'0 |
MeV |
- |
|
- |
|
2.62E-6 |
year |
82.800 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
2,153.300 |
keV |
¹⁸⁶Au |
¹⁸⁶Hg > [ 100 % , β+ , 2,153.3 keV ] > ¹⁸⁶Au |
|
|
α |
5,205.100 |
keV |
¹⁸²Pt |
¹⁸⁶Hg > [ , α , 5,205.1 keV ] > ¹⁸²Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.000'006 |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_187_u |
Unstable |
¹⁸⁷Hg |
Fermion |
80 |
p |
107 |
n |
3/2 |
-1 |
186.969'814'236'0 |
u |
~ 0 |
% |
~ 0 |
-28.117'858'000'0 |
MeV |
7.886'986'000'0 |
MeV |
-1.044'000'000'0 |
nm |
0.450'000'000'0 |
b |
3.49E-6 |
year |
109.980 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
3,865.100 |
keV |
¹⁸⁷Au |
¹⁸⁷Hg > [ 100 % , β+ , 3,865.1 keV ] > ¹⁸⁷Au |
|
|
α |
5,229.700 |
keV |
¹⁸³Pt |
¹⁸⁷Hg > [ , α , 5,229.7 keV ] > ¹⁸³Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁸⁷Os |
0.000'002 |
% |
¹⁷⁹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_188_u |
Unstable |
¹⁸⁸Hg |
Boson |
80 |
p |
108 |
n |
0 |
1 |
187.967'577'049'0 |
u |
~ 0 |
% |
~ 0 |
-30.201'784'000'0 |
MeV |
7.899'051'000'0 |
MeV |
- |
|
- |
|
6.18E-6 |
year |
195.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
1,076.800 |
keV |
¹⁸⁸Au |
¹⁸⁸Hg > [ 100 % , β+ , 1,076.8 keV ] > ¹⁸⁸Au |
|
|
α |
4,705.500 |
keV |
¹⁸⁴Pt |
¹⁸⁸Hg > [ , α , 4,705.5 keV ] > ¹⁸⁴Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁸⁸Os |
? |
% |
¹⁸⁰Hf |
? |
% |
¹⁷⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_189_u |
Unstable |
¹⁸⁹Hg |
Fermion |
80 |
p |
109 |
n |
3/2 |
-1 |
188.968'190'034'0 |
u |
~ 0 |
% |
~ 0 |
-29.630'792'000'0 |
MeV |
7.896'942'000'0 |
MeV |
-0.608'600'000'0 |
nm |
-0.760'000'000'0 |
b |
1.46E-5 |
year |
460.020 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
2,929.000 |
keV |
¹⁸⁹Au |
¹⁸⁹Hg > [ 100 % , β+ , 2,929.0 keV ] > ¹⁸⁹Au |
|
|
α |
4,627.500 |
keV |
¹⁸⁵Pt |
¹⁸⁹Hg > [ , α , 4,627.5 keV ] > ¹⁸⁵Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁸⁹Os |
0.000'060 |
% |
¹⁸⁵Re |
0.000'000 |
% |
¹⁸¹Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_190_u |
Unstable |
¹⁹⁰Hg |
Boson |
80 |
p |
110 |
n |
0 |
1 |
189.966'322'449'0 |
u |
~ 0 |
% |
~ 0 |
-31.370'436'000'0 |
MeV |
7.907'016'000'0 |
MeV |
- |
|
- |
|
3.80E-5 |
year |
1.200 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
ϵ |
1,511.000 |
keV |
¹⁹⁰Au |
¹⁹⁰Hg > [ 100 % , ϵ , 1,511.0 keV ] > ¹⁹⁰Au |
|
|
e+ |
? |
keV |
¹⁹⁰Au |
¹⁹⁰Hg > [ , e+ , ? keV ] > ¹⁹⁰Au |
0.000'000 |
% |
α |
4,069.100 |
keV |
¹⁸⁶Pt |
¹⁹⁰Hg > [ 0.00000034 % , α , 4,069.1 keV ] > ¹⁸⁶Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁹⁰Os |
? |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_191_u |
Unstable |
¹⁹¹Hg |
Fermion |
80 |
p |
111 |
n |
3/2 |
-1 |
190.967'157'105'0 |
u |
~ 0 |
% |
~ 0 |
-30.592'960'000'0 |
MeV |
7.903'805'000'0 |
MeV |
-0.618'000'000'0 |
nm |
-0.800'000'000'0 |
b |
9.19E-5 |
year |
2.902 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
2,194.000 |
keV |
¹⁹¹Au |
¹⁹¹Hg > [ 100 % , β+ , 2,194.0 keV ] > ¹⁹¹Au |
|
|
α |
3,695.100 |
keV |
¹⁸⁷Pt |
¹⁹¹Hg > [ , α , 3,695.1 keV ] > ¹⁸⁷Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁹⁰Os |
? |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_192_u |
Unstable |
¹⁹²Hg |
Boson |
80 |
p |
112 |
n |
0 |
1 |
191.965'634'327'0 |
u |
~ 0 |
% |
~ 0 |
-32.011'418'000'0 |
MeV |
7.912'065'000'0 |
MeV |
- |
|
- |
|
5.55E-4 |
year |
17.500 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
ϵ |
765.100 |
keV |
¹⁹²Au |
¹⁹²Hg > [ 100 % , ϵ , 765.1 keV ] > ¹⁹²Au |
|
|
α |
3,386.600 |
keV |
¹⁸⁸Pt |
¹⁹²Hg > [ , α , 3,386.6 keV ] > ¹⁸⁸Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹²Pt |
0.000'004 |
% |
¹⁸⁸Os |
? |
% |
¹⁸⁰Hf |
? |
% |
¹⁷⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_193_u |
Unstable |
¹⁹³Hg |
Fermion |
80 |
p |
113 |
n |
3/2 |
-1 |
192.966'665'421'0 |
u |
~ 0 |
% |
~ 0 |
-31.050'961'000'0 |
MeV |
7.907'914'000'0 |
MeV |
-0.627'570'000'0 |
nm |
-0.720'000'000'0 |
b |
4.44E-4 |
year |
14.004 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
1,321.200 |
keV |
¹⁹³Au |
¹⁹³Hg > [ 100 % , β+ , 1,321.2 keV ] > ¹⁹³Au |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹³Ir |
94.000'000 |
% |
¹⁸⁹Os |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_194_u |
Unstable |
¹⁹⁴Hg |
Boson |
80 |
p |
114 |
n |
0 |
1 |
193.965'439'409'0 |
u |
~ 0 |
% |
~ 0 |
-32.192'983'000'0 |
MeV |
7.915'7.915'7.915'7.915 |
MeV |
- |
|
- |
|
4.41E+2 |
years |
13.911 |
giga-seconds ( x⁹ ) |
100.000'000 |
% |
ϵ |
69.100 |
keV |
¹⁹⁴Au |
¹⁹⁴Hg > [ 100 % , ϵ , 69.1 keV ] > ¹⁹⁴Au |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹⁴Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_195_u |
Unstable |
¹⁹⁵Hg |
Fermion |
80 |
p |
115 |
n |
1/2 |
-1 |
194.966'720'113'0 |
u |
~ 0 |
% |
~ 0 |
-31.000'015'000'0 |
MeV |
7.909'329'000'0 |
MeV |
0.541'474'900'0 |
nm |
- |
|
1.20E-3 |
year |
37.910 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
547.800 |
keV |
¹⁹⁵Au |
¹⁹⁵Hg > [ 100 % , β+ , 547.8 keV ] > ¹⁹⁵Au |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹⁵Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_196_s |
Stable |
¹⁹⁶Hg |
Boson |
80 |
p |
116 |
n |
0 |
1 |
195.965'832'649'0 |
u |
.0.15 |
% |
#VALUE! |
-31.826'683'000'0 |
MeV |
7.914'373'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
? |
% |
2β+ |
-1,223.630 |
keV |
¹⁹⁶Pt |
¹⁹⁶Hg > [ ? % , 2β+ , -1,223.63 keV ] > ¹⁹⁶Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁹⁶Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_197_u |
Unstable |
¹⁹⁷Hg |
Fermion |
80 |
p |
117 |
n |
1/2 |
-1 |
196.967'212'908'0 |
u |
~ 0 |
% |
~ 0 |
-30.540'979'000'0 |
MeV |
7.908'643'000'0 |
MeV |
0.527'374'400'0 |
nm |
- |
|
7.41E-3 |
year |
233.798 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
ϵ |
600.110 |
keV |
¹⁹⁷Au |
¹⁹⁷Hg > [ 100 % , ϵ , 600.11 keV ] > ¹⁹⁷Au |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹⁷Au |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_198_s |
Stable |
¹⁹⁸Hg |
Boson |
80 |
p |
118 |
n |
0 |
1 |
197.966'769'032'0 |
u |
9.970'000 |
% |
19.737'286'872'5 |
-30.954'447'000'0 |
MeV |
7.911'553'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_199_s |
Stable |
¹⁹⁹Hg |
Fermion |
80 |
p |
119 |
n |
1/2 |
-1 |
198.968'279'932'0 |
u |
16.870'000 |
% |
33.565'948'824'5 |
-29.547'053'000'0 |
MeV |
7.905'284'000'0 |
MeV |
0.505'885'500'0 |
nm |
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_200_s |
Stable |
²⁰⁰Hg |
Boson |
80 |
p |
120 |
n |
0 |
1 |
199.968'326'004'0 |
u |
23.000'000 |
% |
45.992'714'980'9 |
-29.504'137'000'0 |
MeV |
7.905'899'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_201_s |
Stable |
²⁰¹Hg |
Fermion |
80 |
p |
121 |
n |
3/2 |
-1 |
200.970'302'268'0 |
u |
13.180'000 |
% |
26.487'885'838'9 |
-27.663'259'000'0 |
MeV |
7.897'564'000'0 |
MeV |
-0.560'225'700'0 |
nm |
0.385'000'000'0 |
b |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_202_s |
Stable |
²⁰²Hg |
Boson |
80 |
p |
122 |
n |
0 |
1 |
201.970'643'011'0 |
u |
29.860'000 |
% |
60.308'434'003'1 |
-27.345'859'000'0 |
MeV |
7.896'852'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_203_u |
Unstable |
²⁰³Hg |
Fermion |
80 |
p |
123 |
n |
5/2 |
-1 |
202.972'872'484'0 |
u |
~ 0 |
% |
~ 0 |
-25.269'119'000'0 |
MeV |
7.887'482'000'0 |
MeV |
0.848'950'000'0 |
nm |
0.343'000'000'0 |
b |
1.28E-1 |
year |
4.027 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
β- |
492.070 |
keV |
²⁰³Tl |
²⁰³Hg > [ 100 % , β- , 492.07 keV ] > ²⁰³Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰³Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_204_s |
Stable |
²⁰⁴Hg |
Boson |
80 |
p |
124 |
n |
0 |
1 |
203.973'493'933'0 |
u |
6.870'000 |
% |
14.012'979'033'2 |
-24.690'242'000'0 |
MeV |
7.885'545'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
? |
% |
2β- |
419.490 |
keV |
²⁰⁴Pb |
²⁰⁴Hg > [ ? % , 2β- , 419.49 keV ] > ²⁰⁴Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
²⁰⁰Hg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_205_u |
Unstable |
²⁰⁵Hg |
Fermion |
80 |
p |
125 |
n |
1/2 |
-1 |
204.976'073'386'0 |
u |
~ 0 |
% |
~ 0 |
-22.287'497'000'0 |
MeV |
-22.287'497'000'0 |
MeV |
0.601'000'000'0 |
nm |
- |
|
9.82E-6 |
year |
310.020 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
1,533.090 |
keV |
²⁰⁵Tl |
²⁰⁵Hg > [ 100 % , β- , 1,533.09 keV ] > ²⁰⁵Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰⁵Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_206_u |
Unstable |
²⁰⁶Hg |
Boson |
80 |
p |
126 |
n |
0 |
1 |
205.977'514'066'0 |
u |
~ 0 |
% |
~ 0 |
-20.945'512'000'0 |
MeV |
7.869'170'000'0 |
MeV |
- |
|
- |
|
1.55E-5 |
year |
489.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
1,307.600 |
keV |
²⁰⁶Tl |
²⁰⁶Hg > [ 100 % , β- , 1,307.6 keV ] > ²⁰⁶Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰⁶Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_207_u |
Unstable |
²⁰⁷Hg |
Fermion |
80 |
p |
127 |
n |
9/2 |
1 |
206.982'588'545'0 |
u |
~ 0 |
% |
~ 0 |
-16.218'666'000'0 |
MeV |
7.847'312'000'0 |
MeV |
- |
|
- |
|
5.39E-6 |
year |
169.980 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
4,815.000 |
keV |
²⁰⁷Tl |
²⁰⁷Hg > [ 100 % , β- , 4,815.0 keV ] > ²⁰⁷Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰⁷Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_208_u |
Unstable |
²⁰⁸Hg |
Boson |
80 |
p |
128 |
n |
0 |
1 |
207.985'940'000'0 |
u |
~ 0 |
% |
~ 0 |
-13.097'000'000'0 |
MeV |
7.833'000'000'0 |
MeV |
- |
|
- |
|
7.92E-5 |
year |
2.498 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
3,653.000 |
keV |
²⁰⁸Tl |
²⁰⁸Hg > [ 100 % , β- , 3,653.0 keV ] > ²⁰⁸Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰⁸Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_209_u |
Unstable |
²⁰⁹Hg |
Fermion |
80 |
p |
129 |
n |
9/2 |
1 |
208.991'040'000'0 |
u |
~ 0 |
% |
~ 0 |
-8.346'000'000'0 |
MeV |
7.812'000'000'0 |
MeV |
- |
|
- |
|
1.17E-6 |
year |
37.020 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
5,292.000 |
keV |
²⁰⁹Tl |
²⁰⁹Hg > [ 100 % , β- , 5,292.0 keV ] > ²⁰⁹Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰⁵Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_080_hg_210_u |
Unstable |
²¹⁰Hg |
Boson |
80 |
p |
130 |
n |
0 |
1 |
209.994'510'000'0 |
u |
~ 0 |
% |
~ 0 |
-5.114'000'000'0 |
MeV |
7.798'000'000'0 |
MeV |
- |
|
- |
|
1.90E-5 |
year |
600.000 |
seconds ( x⁰ ) |
? |
% |
β- |
4,132.000 |
keV |
²¹⁰Tl |
²¹⁰Hg > [ ? % , β- , 4,132.0 keV ] > ²¹⁰Tl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
²⁰⁵Tl |
? |
% |
²⁰⁶Pb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|