| Isotope_074_w_158_u |
Unstable |
¹⁵⁸W |
Boson |
74 |
p |
84 |
n |
0 |
1 |
157.974'562'000'0 |
u |
~ 0 |
% |
~ 0 |
-23.695'000'000'0 |
MeV |
7.855'000'000'0 |
MeV |
- |
|
- |
|
4.34E-11 |
year |
1.370 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
α |
6,612.650 |
keV |
¹⁵⁴Hf |
¹⁵⁸W > [ 100 % , α , 6,612.65 keV ] > ¹⁵⁴Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁴²Nd |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁴Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_159_u |
Unstable |
¹⁵⁹W |
Fermion |
74 |
p |
85 |
n |
7/2 |
-1 |
158.972'918'000'0 |
u |
~ 0 |
% |
~ 0 |
-25.227'000'000'0 |
MeV |
7.866'000'000'0 |
MeV |
- |
|
- |
|
2.60E-10 |
year |
8.200 |
milli-seconds ( x⁻³ ) |
82.000'000 |
% |
α |
6,450.410 |
keV |
¹⁵⁵Hf |
¹⁵⁹W > [ 82 % , α , 6,450.41 keV ] > ¹⁵⁵Hf |
|
|
β+ |
8,200.000 |
keV |
¹⁵⁹Ta |
¹⁵⁹W > [ , β+ , 8,200.0 keV ] > ¹⁵⁹Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
53.132'851 |
% |
¹⁵¹Eu |
19.032'616 |
% |
¹⁴³Nd |
? |
% |
¹⁵⁹Tb |
? |
% |
¹⁴²Nd |
? |
% |
¹⁵⁵Gd |
? |
% |
¹⁵⁰Sm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_160_u |
Unstable |
¹⁶⁰W |
Boson |
74 |
p |
86 |
n |
0 |
1 |
159.968'478'805'0 |
u |
~ 0 |
% |
~ 0 |
-29.361'804'000'0 |
MeV |
7.892'993'000'0 |
MeV |
- |
|
- |
|
2.85E-9 |
year |
90.000 |
milli-seconds ( x⁻³ ) |
87.000'000 |
% |
α |
6,065.450 |
keV |
¹⁵⁶Hf |
¹⁶⁰W > [ 87 % , α , 6,065.45 keV ] > ¹⁵⁶Hf |
|
|
β+ |
5,492.000 |
keV |
¹⁶⁰Ta |
¹⁶⁰W > [ , β+ , 5,492.0 keV ] > ¹⁶⁰Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7.426'320 |
% |
¹⁵⁶Hf |
? |
% |
¹⁴³Nd |
? |
% |
¹⁵¹Eu |
? |
% |
¹⁶⁰Dy |
? |
% |
¹⁵²Sm |
? |
% |
¹⁵⁶Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_161_u |
Unstable |
¹⁶¹W |
Fermion |
74 |
p |
87 |
n |
7/2 |
-1 |
160.967'357'000'0 |
u |
~ 0 |
% |
~ 0 |
-30.407'000'000'0 |
MeV |
7.901'000'000'0 |
MeV |
- |
|
- |
|
1.30E-8 |
year |
409.000 |
milli-seconds ( x⁻³ ) |
73.000'000 |
% |
α |
5,922.800 |
keV |
¹⁵⁷Hf |
¹⁶¹W > [ 73 % , α , 5,922.8 keV ] > ¹⁵⁷Hf |
|
|
β+ |
7,305.000 |
keV |
¹⁶¹Ta |
¹⁶¹W > [ , β+ , 7,305.0 keV ] > ¹⁶¹Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.875'285 |
% |
¹⁴⁵Nd |
0.000'442 |
% |
¹⁴⁰Ce |
0.000'000 |
% |
¹⁴¹Pr |
? |
% |
¹⁵⁷Gd |
? |
% |
¹⁵³Eu |
? |
% |
¹⁶¹Dy |
? |
% |
¹⁵²Sm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_162_u |
Unstable |
¹⁶²W |
Boson |
74 |
p |
88 |
n |
0 |
1 |
161.963'497'417'0 |
u |
~ 0 |
% |
~ 0 |
-34.001'937'000'0 |
MeV |
7.923'837'000'0 |
MeV |
- |
|
- |
|
4.31E-8 |
year |
1.360 |
seconds ( x⁰ ) |
54.800'000 |
% |
β+ |
4,758.200 |
keV |
¹⁶²Ta |
¹⁶²W > [ 54.8 % , β+ , 4,758.2 keV ] > ¹⁶²Ta |
|
|
α |
5,677.270 |
keV |
¹⁵⁸Hf |
¹⁶²W > [ , α , 5,677.27 keV ] > ¹⁵⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20.577'339 |
% |
¹⁴²Nd |
? |
% |
¹⁵⁴Gd |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁸Gd |
? |
% |
¹⁶²Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_163_u |
Unstable |
¹⁶³W |
Fermion |
74 |
p |
89 |
n |
3/2 |
-1 |
162.962'523'542'0 |
u |
~ 0 |
% |
~ 0 |
-34.909'095'000'0 |
MeV |
7.930'308'000'0 |
MeV |
- |
|
- |
|
8.87E-8 |
year |
2.800 |
seconds ( x⁰ ) |
87.000'000 |
% |
β+ |
6,609.800 |
keV |
¹⁶³Ta |
¹⁶³W > [ 87 % , β+ , 6,609.8 keV ] > ¹⁶³Ta |
|
|
α |
5,519.500 |
keV |
¹⁵⁹Hf |
¹⁶³W > [ , α , 5,519.5 keV ] > ¹⁵⁹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
87.000'000 |
% |
¹⁶³Dy |
8.624'087 |
% |
¹⁵⁹Tb |
2.988'954 |
% |
¹⁵¹Eu |
1.070'637 |
% |
¹⁴³Nd |
0.499'451 |
% |
¹⁵⁵Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_164_u |
Unstable |
¹⁶⁴W |
Boson |
74 |
p |
90 |
n |
0 |
1 |
163.958'954'382'0 |
u |
~ 0 |
% |
~ 0 |
-38.233'747'000'0 |
MeV |
7.951'440'000'0 |
MeV |
- |
|
- |
|
2.00E-7 |
year |
6.300 |
seconds ( x⁰ ) |
96.200'000 |
% |
β+ |
4,026.900 |
keV |
¹⁶⁴Ta |
¹⁶⁴W > [ 96.2 % , β+ , 4,026.9 keV ] > ¹⁶⁴Ta |
|
|
α |
4,026.900 |
keV |
¹⁶⁰Hf |
¹⁶⁴W > [ , α , 4,026.9 keV ] > ¹⁶⁰Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.773'400 |
% |
¹⁶⁰Dy |
0.000'248 |
% |
¹⁴⁰Ce |
? |
% |
¹⁵²Sm |
? |
% |
¹⁵⁶Gd |
? |
% |
¹⁶⁴Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_165_u |
Unstable |
¹⁶⁵W |
Fermion |
74 |
p |
91 |
n |
3/2 |
-1 |
164.950'772'514'0 |
u |
~ 0 |
% |
~ 0 |
-38.861'977'000'0 |
MeV |
7.955'974'000'0 |
MeV |
- |
|
- |
|
1.62E-7 |
year |
5.100 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
5,970.900 |
keV |
¹⁶⁵Ta |
¹⁶⁵W > [ 100 % , β+ , 5,970.9 keV ] > ¹⁶⁵Ta |
|
|
α |
5,031.800 |
keV |
¹⁶¹Hf |
¹⁶⁵W > [ , α , 5,031.8 keV ] > ¹⁶¹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶⁵Ho |
0.200'000 |
% |
¹⁶¹Dy |
0.000'259 |
% |
¹⁵⁷Gd |
0.000'001 |
% |
¹⁵³Eu |
0.000'000 |
% |
¹⁴⁵Nd |
0.000'000 |
% |
¹⁴¹Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_166_u |
Unstable |
¹⁶⁶W |
Boson |
74 |
p |
92 |
n |
0 |
1 |
165.950'512'000'0 |
u |
~ 0 |
% |
~ 0 |
-41.891'844'000'0 |
MeV |
7.974'921'000'0 |
MeV |
- |
|
- |
|
6.08E-7 |
year |
19.200 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
3,183.700 |
keV |
¹⁶⁶Ta |
¹⁶⁶W > [ 100 % , β+ , 3,183.7 keV ] > ¹⁶⁶Ta |
|
|
α |
4,856.340 |
keV |
¹⁶²Hf |
¹⁶⁶W > [ , α , 4,856.34 keV ] > ¹⁶²Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶⁶Er |
0.000'000 |
% |
¹⁴²Nd |
? |
% |
¹⁵⁴Gd |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁸Gd |
? |
% |
¹⁶²Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_167_u |
Unstable |
¹⁶⁷W |
Fermion |
74 |
p |
93 |
n |
3/2 |
-1 |
166.948'093'000'0 |
u |
~ 0 |
% |
~ 0 |
-42.088'612'000'0 |
MeV |
7.976'676'000'0 |
MeV |
- |
|
- |
|
6.31E-7 |
year |
19.900 |
seconds ( x⁰ ) |
99.960'000 |
% |
β+ |
5,240.200 |
keV |
¹⁶⁷Ta |
¹⁶⁷W > [ 99.96 % , β+ , 5,240.2 keV ] > ¹⁶⁷Ta |
|
|
α |
4,772.800 |
keV |
¹⁶³Hf |
¹⁶⁷W > [ , α , 4,772.8 keV ] > ¹⁶³Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
99.960'000 |
% |
¹⁶⁷Er |
0.040'000 |
% |
¹⁶³Dy |
0.000'000 |
% |
¹⁵⁹Tb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_168_u |
Unstable |
¹⁶⁸W |
Boson |
74 |
p |
94 |
n |
0 |
1 |
167.951'808'394'0 |
u |
~ 0 |
% |
~ 0 |
-44.890'192'000'0 |
MeV |
7.993'916'000'0 |
MeV |
- |
|
- |
|
1.62E-6 |
year |
51.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
2,481.500 |
keV |
¹⁶⁸Ta |
¹⁶⁸W > [ 100 % , β+ , 2,481.5 keV ] > ¹⁶⁸Ta |
|
|
α |
4,506.400 |
keV |
¹⁶⁴Hf |
¹⁶⁸W > [ , α , 4,506.4 keV ] > ¹⁶⁴Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁶⁸Er |
? |
% |
¹⁶⁴Dy |
? |
% |
¹⁶⁰Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_169_u |
Unstable |
¹⁶⁹W |
Fermion |
74 |
p |
95 |
n |
5/2 |
-1 |
168.951'778'790'0 |
u |
~ 0 |
% |
~ 0 |
-44.917'768'000'0 |
MeV |
7.994'537'000'0 |
MeV |
- |
|
- |
|
2.41E-6 |
year |
76.200 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
4,350.500 |
keV |
¹⁶⁹Ta |
¹⁶⁹W > [ 100 % , β+ , 4,350.5 keV ] > ¹⁶⁹Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶⁹Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_170_u |
Unstable |
¹⁷⁰W |
Boson |
74 |
p |
96 |
n |
0 |
1 |
169.949'228'482'0 |
u |
~ 0 |
% |
~ 0 |
-47.293'364'000'0 |
MeV |
8.008'962'000'0 |
MeV |
- |
|
- |
|
4.60E-6 |
year |
145.020 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
1,822.100 |
keV |
¹⁷⁰Ta |
¹⁷⁰W > [ 100 % , β+ , 1,822.1 keV ] > ¹⁷⁰Ta |
|
|
α |
4,140.700 |
keV |
¹⁶⁶Hf |
¹⁷⁰W > [ , α , 4,140.7 keV ] > ¹⁶⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁰Yb |
1.000'000 |
% |
¹⁶⁸Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_171_u |
Unstable |
¹⁷¹W |
Fermion |
74 |
p |
97 |
n |
5/2 |
-1 |
170.949'451'000'0 |
u |
~ 0 |
% |
~ 0 |
-47.086'091'000'0 |
MeV |
8.008'115'000'0 |
MeV |
- |
|
- |
|
4.53E-6 |
year |
142.980 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
3,612.000 |
keV |
¹⁷¹Ta |
¹⁷¹W > [ 100 % , β+ , 3,612.0 keV ] > ¹⁷¹Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷¹Yb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_172_u |
Unstable |
¹⁷²W |
Boson |
74 |
p |
98 |
n |
0 |
1 |
171.947'292'000'0 |
u |
~ 0 |
% |
~ 0 |
-49.097'186'000'0 |
MeV |
8.020'175'000'0 |
MeV |
- |
|
- |
|
1.27E-5 |
year |
400.200 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
1,210.600 |
keV |
¹⁷²Ta |
¹⁷²W > [ 100 % , β+ , 1,210.6 keV ] > ¹⁷²Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷²Yb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_173_u |
Unstable |
¹⁷³W |
Fermion |
74 |
p |
99 |
n |
5/2 |
-1 |
172.947'689'000'0 |
u |
~ 0 |
% |
~ 0 |
-48.727'383'000'0 |
MeV |
8.018'333'000'0 |
MeV |
- |
|
- |
|
1.46E-5 |
year |
460.020 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
2,647.000 |
keV |
¹⁷³Ta |
¹⁷³W > [ 100 % , β+ , 2,647.0 keV ] > ¹⁷³Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷³Yb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_174_u |
Unstable |
¹⁷⁴W |
Boson |
74 |
p |
100 |
n |
0 |
1 |
173.946'079'000'0 |
u |
~ 0 |
% |
~ 0 |
-50.227'088'000'0 |
MeV |
8.027'256'000'0 |
MeV |
- |
|
- |
|
6.31E-5 |
year |
1.990 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
491.500 |
keV |
¹⁷⁴Ta |
¹⁷⁴W > [ 100 % , β+ , 491.5 keV ] > ¹⁷⁴Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁰Yb |
? |
% |
¹⁷⁴Yb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_175_u |
Unstable |
¹⁷⁵W |
Fermion |
74 |
p |
101 |
n |
1/2 |
-1 |
174.946'717'000'0 |
u |
~ 0 |
% |
~ 0 |
-49.632'795'000'0 |
MeV |
8.024'112'000'0 |
MeV |
- |
|
- |
|
6.69E-5 |
year |
2.110 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
1,753.700 |
keV |
¹⁷⁵Ta |
¹⁷⁵W > [ 100 % , β+ , 1,753.7 keV ] > ¹⁷⁵Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁵Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_176_u |
Unstable |
¹⁷⁶W |
Boson |
74 |
p |
102 |
n |
0 |
1 |
175.945'634'000'0 |
u |
~ 0 |
% |
~ 0 |
-50.641'603'000'0 |
MeV |
8.030'112'000'0 |
MeV |
- |
|
- |
|
2.85E-4 |
year |
9.000 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
ϵ |
723.800 |
keV |
¹⁷⁶Ta |
¹⁷⁶W > [ 100 % , ϵ , 723.8 keV ] > ¹⁷⁶Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_177_u |
Unstable |
¹⁷⁷W |
Fermion |
74 |
p |
103 |
n |
1/2 |
-1 |
176.946'643'000'0 |
u |
~ 0 |
% |
~ 0 |
-49.701'726'000'0 |
MeV |
8.025'035'000'0 |
MeV |
- |
|
- |
|
2.51E-4 |
year |
7.920 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
999.700 |
keV |
¹⁷⁷Ta |
¹⁷⁷W > [ 100 % , β+ , 999.7 keV ] > ¹⁷⁷Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁷Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_178_u |
Unstable |
¹⁷⁸W |
Boson |
74 |
p |
104 |
n |
0 |
1 |
177.945'876'236'0 |
u |
~ 0 |
% |
~ 0 |
-50.415'962'000'0 |
MeV |
8.029'308'000'0 |
MeV |
- |
|
- |
|
5.92E-2 |
year |
1.870 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
ϵ |
91.300 |
keV |
¹⁷⁸Ta |
¹⁷⁸W > [ 100 % , ϵ , 91.3 keV ] > ¹⁷⁸Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_179_u |
Unstable |
¹⁷⁹W |
Fermion |
74 |
p |
105 |
n |
7/2 |
-1 |
178.947'070'447'0 |
u |
~ 0 |
% |
~ 0 |
-49.303'561'000'0 |
MeV |
8.023'328'000'0 |
MeV |
- |
|
- |
|
4.23E-3 |
year |
133.380 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
40.600 |
keV |
¹⁷⁹Ta |
¹⁷⁹W > [ 100 % , β+ , 40.6 keV ] > ¹⁷⁹Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_180_s |
Stable |
¹⁸⁰W |
Boson |
74 |
p |
106 |
n |
0 |
1 |
179.946'704'459'0 |
u |
0.120'000 |
% |
0.215'936'045'4 |
-49.644'477'000'0 |
MeV |
8.025'488'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
? |
% |
α |
2,508.120 |
keV |
¹⁷⁶Hf |
¹⁸⁰W > [ ? % , α , 2,508.12 keV ] > ¹⁷⁶Hf |
|
|
2β+ |
-1,900.480 |
keV |
¹⁸⁰Hf |
¹⁸⁰W > [ , 2β+ , -1,900.48 keV ] > ¹⁸⁰Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁸⁰Hf |
? |
% |
¹⁷⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_181_u |
Unstable |
¹⁸¹W |
Fermion |
74 |
p |
107 |
n |
9/2 |
1 |
180.948'197'248'0 |
u |
~ 0 |
% |
~ 0 |
-48.253'952'000'0 |
MeV |
8.018'059'000'0 |
MeV |
- |
|
- |
|
3.32E-1 |
year |
10.470 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
ϵ |
187.680 |
keV |
¹⁸¹Ta |
¹⁸¹W > [ 100 % , ϵ , 187.68 keV ] > ¹⁸¹Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁸¹Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_182_s |
Stable |
¹⁸²W |
Boson |
74 |
p |
108 |
n |
0 |
1 |
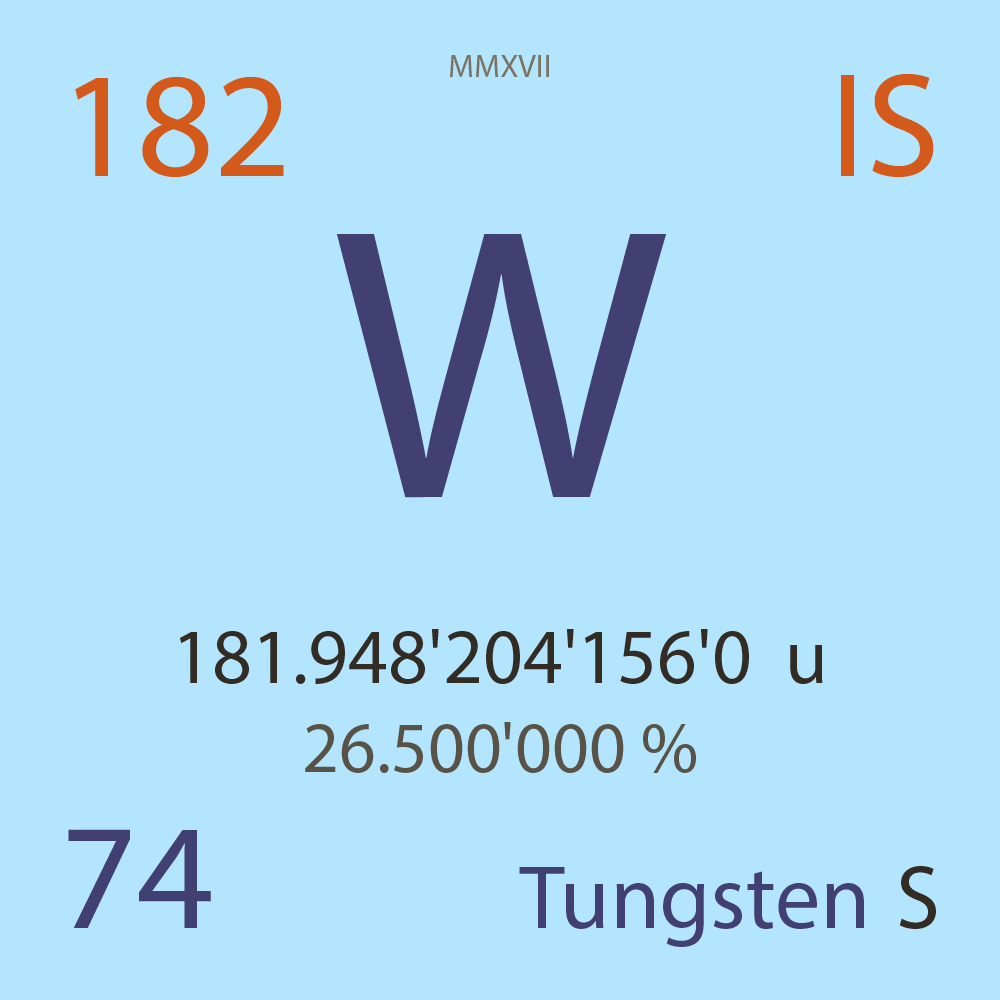
181.948'204'156'0 |
u |
26.500'000 |
% |
48.216'274'101'3 |
-48.247'518'000'0 |
MeV |
8.018'316'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
? |
% |
α |
1,771.830 |
keV |
¹⁷⁸Hf |
¹⁸²W > [ ? % , α , 1,771.83 keV ] > ¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_183_s |
Stable |
¹⁸³W |
Fermion |
74 |
p |
109 |
n |
1/2 |
-1 |
182.950'222'951'0 |
u |
14.310'000 |
% |
26.180'176'904'3 |
-46.367'023'000'0 |
MeV |
8.008'330'000'0 |
MeV |
0.117'784'760'0 |
nm |
- |
|
- |
|
|
|
? |
% |
α |
1,680.000 |
keV |
¹⁷⁹Hf |
¹⁸³W > [ ? % , α , 1,680.0 keV ] > ¹⁷⁹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁷⁹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
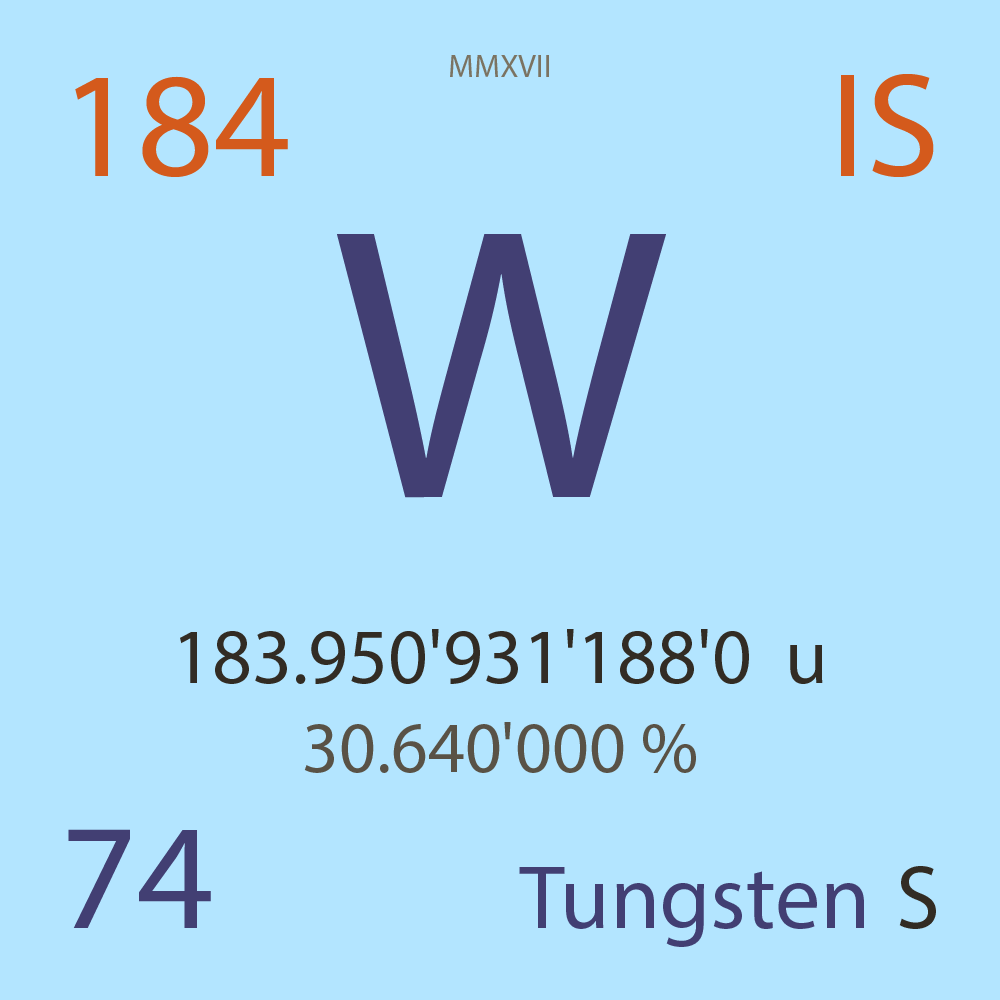
| Isotope_074_w_184_s |
Stable |
¹⁸⁴W |
Boson |
74 |
p |
110 |
n |
0 |
1 |
183.950'931'188'0 |
u |
30.640'000 |
% |
56.362'565'316'0 |
-45.707'304'000'0 |
MeV |
8.005'087'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
? |
% |
α |
1,656.180 |
keV |
¹⁸⁰Hf |
¹⁸⁴W > [ ? % , α , 1,656.18 keV ] > ¹⁸⁰Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁸⁰Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_185_u |
Unstable |
¹⁸⁵W |
Fermion |
74 |
p |
111 |
n |
3/2 |
-1 |
184.953'419'264'0 |
u |
~ 0 |
% |
~ 0 |
-43.389'676'000'0 |
MeV |
7.992'917'000'0 |
MeV |
- |
|
- |
|
2.06E-1 |
year |
6.490 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
β- |
432.476 |
keV |
¹⁸⁵Re |
¹⁸⁵W > [ 100 % , β- , 432.476 keV ] > ¹⁸⁵Re |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁸⁵Re |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
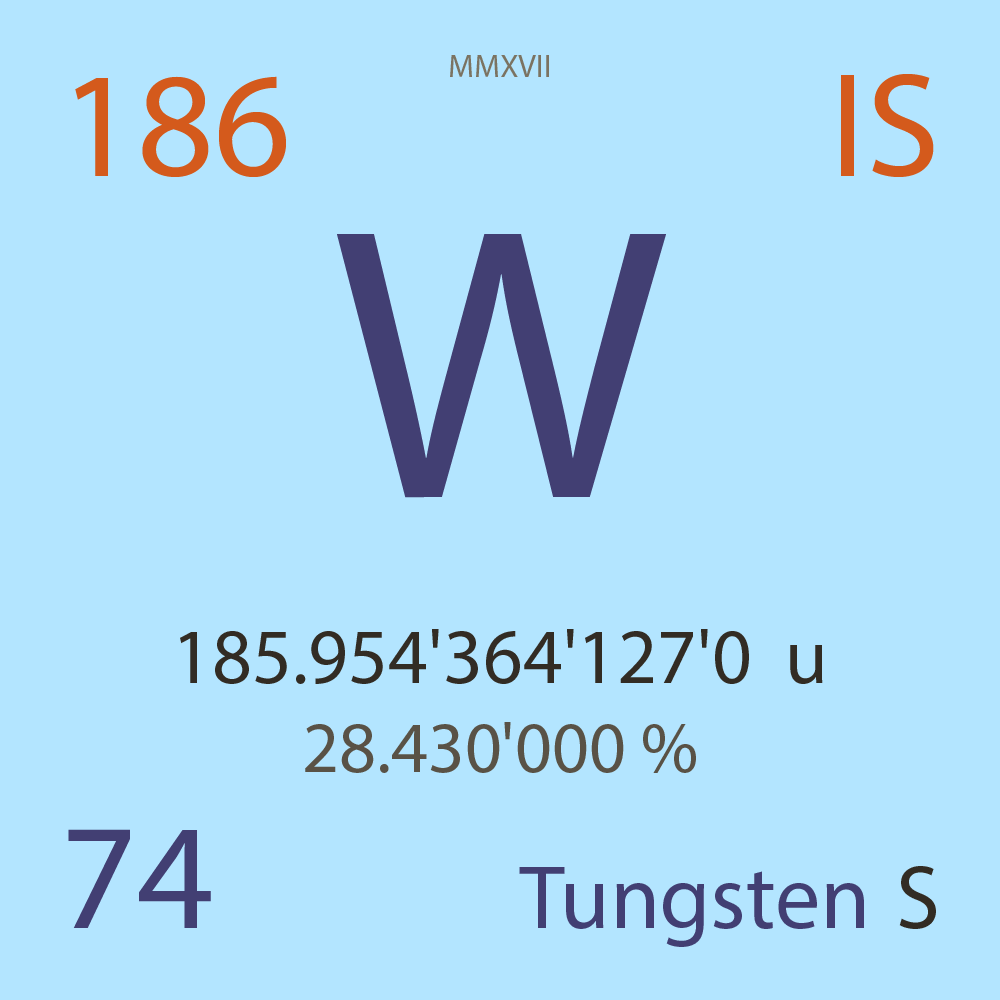
| Isotope_074_w_186_s |
Stable |
¹⁸⁶W |
Boson |
74 |
p |
112 |
n |
0 |
1 |
185.954'364'127'0 |
u |
28.430'000 |
% |
52.866'825'721'3 |
-42.509'542'000'0 |
MeV |
7.988'607'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
? |
% |
2β- |
489.940 |
keV |
¹⁸⁶Os |
¹⁸⁶W > [ ? % , 2β- , 489.94 keV ] > ¹⁸⁶Os |
|
|
α |
1,124.110 |
keV |
¹⁷⁸Hf |
¹⁸⁶W > [ , α , 1,124.11 keV ] > ¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_187_u |
Unstable |
¹⁸⁷W |
Fermion |
74 |
p |
113 |
n |
3/2 |
-1 |
186.957'160'466'0 |
u |
~ 0 |
% |
~ 0 |
-39.904'768'000'0 |
MeV |
7.975'120'000'0 |
MeV |
0.621'000'000'0 |
nm |
- |
|
2.71E-3 |
year |
85.390 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
1,310.940 |
keV |
¹⁸⁷Re |
¹⁸⁷W > [ 100 % , β- , 1,310.94 keV ] > ¹⁸⁷Re |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁸⁷Os |
? |
% |
¹⁷⁹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_188_u |
Unstable |
¹⁸⁸W |
Boson |
74 |
p |
114 |
n |
0 |
1 |
187.958'489'105'0 |
u |
~ 0 |
% |
~ 0 |
-38.667'150'000'0 |
MeV |
7.969'048'000'0 |
MeV |
- |
|
- |
|
1.91E-1 |
year |
6.029 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
β- |
349.000 |
keV |
¹⁸⁸Re |
¹⁸⁸W > [ 100 % , β- , 349.0 keV ] > ¹⁸⁸Re |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁸⁸Os |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_189_u |
Unstable |
¹⁸⁹W |
Fermion |
74 |
p |
115 |
n |
3/2 |
-1 |
188.961'912'868'0 |
u |
~ 0 |
% |
~ 0 |
-35.477'935'000'0 |
MeV |
7.952'715'000'0 |
MeV |
- |
|
- |
|
2.21E-5 |
year |
696.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
2,500.000 |
keV |
¹⁸⁹Re |
¹⁸⁹W > [ 100 % , β- , 2,500.0 keV ] > ¹⁸⁹Re |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁸⁹Os |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_190_u |
Unstable |
¹⁹⁰W |
Boson |
74 |
p |
116 |
n |
0 |
1 |
189.963'181'378'0 |
u |
~ 0 |
% |
~ 0 |
-34.296'326'000'0 |
MeV |
7.947'121'000'0 |
MeV |
- |
|
- |
|
5.70E-5 |
year |
1.800 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
1,270.000 |
keV |
¹⁹⁰Re |
¹⁹⁰W > [ 100 % , β- , 1,270.0 keV ] > ¹⁹⁰Re |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹⁰Os |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_191_u |
Unstable |
¹⁹¹W |
Fermion |
74 |
p |
117 |
n |
3/2 |
-1 |
190.966'600'000'0 |
u |
~ 0 |
% |
~ 0 |
-31.112'000'000'0 |
MeV |
7.931'000'000'0 |
MeV |
- |
|
- |
|
6.34E-7 |
year |
20.000 |
seconds ( x⁰ ) |
? |
% |
β- |
3,237.000 |
keV |
¹⁹¹Re |
¹⁹¹W > [ ? % , β- , 3,237.0 keV ] > ¹⁹¹Re |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁹¹Ir |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_074_w_192_u |
Unstable |
¹⁹²W |
Boson |
74 |
p |
118 |
n |
0 |
1 |
191.968'170'000'0 |
u |
~ 0 |
% |
~ 0 |
-29.649'000'000'0 |
MeV |
7.924'000'000'0 |
MeV |
- |
|
- |
|
3.17E-7 |
year |
10.000 |
seconds ( x⁰ ) |
? |
% |
β- |
2,059.000 |
keV |
¹⁹²Re |
¹⁹²W > [ ? % , β- , 2,059.0 keV ] > ¹⁹²Re |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁸⁸Os |
? |
% |
¹⁹²Pt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|