| Isotope_073_ta_155_u |
Unstable |
¹⁵⁵Ta |
Boson |
73 |
p |
82 |
n |
11/2 |
-1 |
154.974'592'000'0 |
u |
~ 0 |
% |
~ 0 |
-23.668'000'000'0 |
MeV |
7.856'000'000'0 |
MeV |
- |
|
- |
|
4.12E-13 |
year |
13.000 |
micro-seconds ( x⁻⁶ ) |
100.000'000 |
% |
p |
1,776.000 |
keV |
¹⁵⁴Hf |
¹⁵⁵Ta > [ 100 % , p , 1,776.0 keV ] > ¹⁵⁴Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁴²Nd |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁴Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
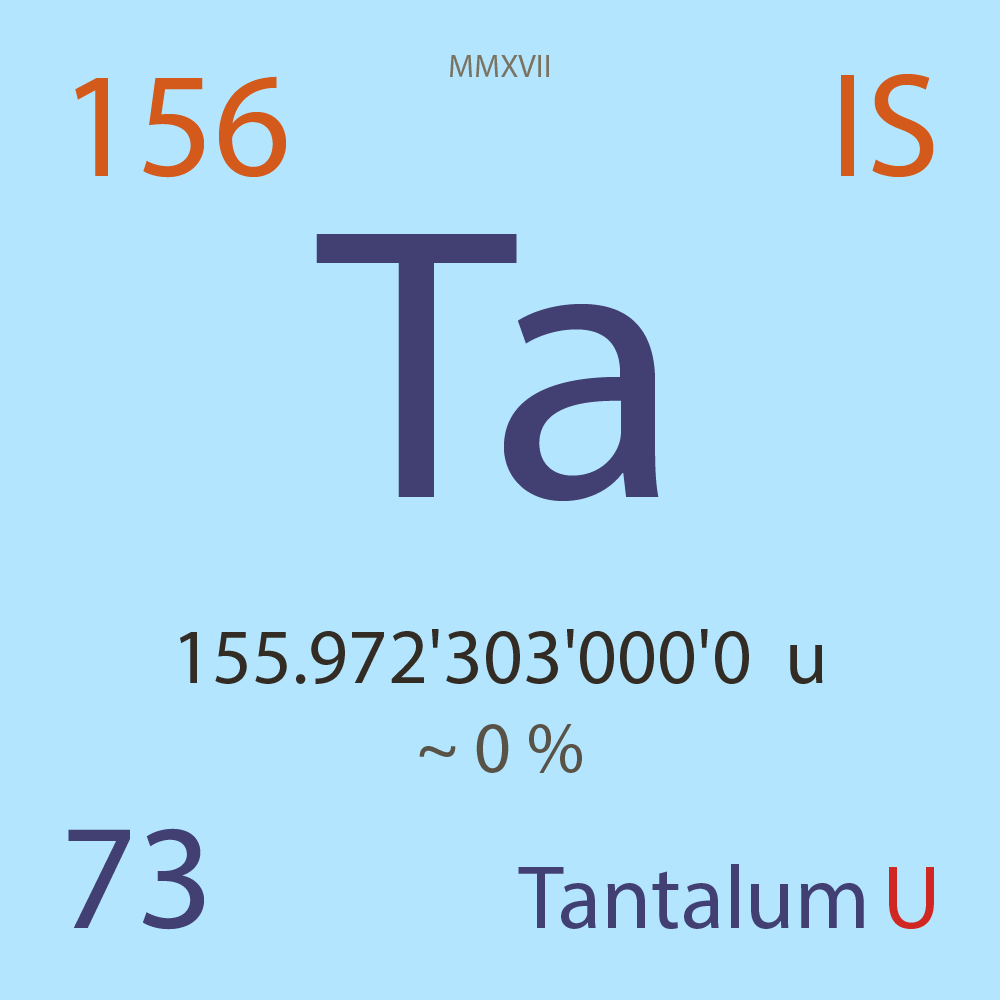
| Isotope_073_ta_156_u |
Unstable |
¹⁵⁶Ta |
Fermion |
73 |
p |
83 |
n |
2 |
-1 |
155.972'303'000'0 |
u |
~ 0 |
% |
~ 0 |
-25.799'000'000'0 |
MeV |
7.871'000'000'0 |
MeV |
- |
|
- |
|
4.56E-12 |
year |
144.000 |
micro-seconds ( x⁻⁶ ) |
100.000'000 |
% |
p |
1,013.600 |
keV |
¹⁵⁵Hf |
¹⁵⁶Ta > [ 100 % , p , 1,013.6 keV ] > ¹⁵⁵Hf |
|
|
β+ |
11,031.000 |
keV |
¹⁵⁶Hf |
¹⁵⁶Ta > [ , β+ , 11,031.0 keV ] > ¹⁵⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
64.796'160 |
% |
¹⁵¹Eu |
23.210'507 |
% |
¹⁴³Nd |
? |
% |
¹⁴²Nd |
? |
% |
¹⁵⁵Gd |
? |
% |
¹⁴⁰Ce |
? |
% |
¹⁵²Sm |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁶Gd |
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_157_u |
Unstable |
¹⁵⁷Ta |
Boson |
73 |
p |
84 |
n |
1/2 |
1 |
156.968'192'445'0 |
u |
~ 0 |
% |
~ 0 |
-29.628'547'000'0 |
MeV |
7.896'268'000'0 |
MeV |
- |
|
- |
|
3.20E-13 |
year |
10.100 |
micro-seconds ( x⁻⁶ ) |
96.000'000 |
% |
α |
6,354.520 |
keV |
¹⁵³Lu |
¹⁵⁷Ta > [ 96 % , α , 6,354.52 keV ] > ¹⁵³Lu |
|
|
p |
934.650 |
keV |
¹⁵⁶Hf |
¹⁵⁷Ta > [ , p , 934.65 keV ] > ¹⁵⁶Hf |
1.000'000 |
% |
β+ |
8,104.000 |
keV |
¹⁵⁷Hf |
¹⁵⁷Ta > [ 1 % , β+ , 8,104.0 keV ] > ¹⁵⁷Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12.740'353 |
% |
¹⁴⁵Nd |
0.290'433 |
% |
¹⁴⁰Ce |
0.000'000 |
% |
¹⁴¹Pr |
? |
% |
¹⁴³Nd |
? |
% |
¹⁵⁷Gd |
? |
% |
¹⁵³Eu |
? |
% |
¹⁵¹Eu |
? |
% |
¹⁵²Sm |
? |
% |
¹⁵⁶Gd |
|
|
|
|
|
|
|
|
|
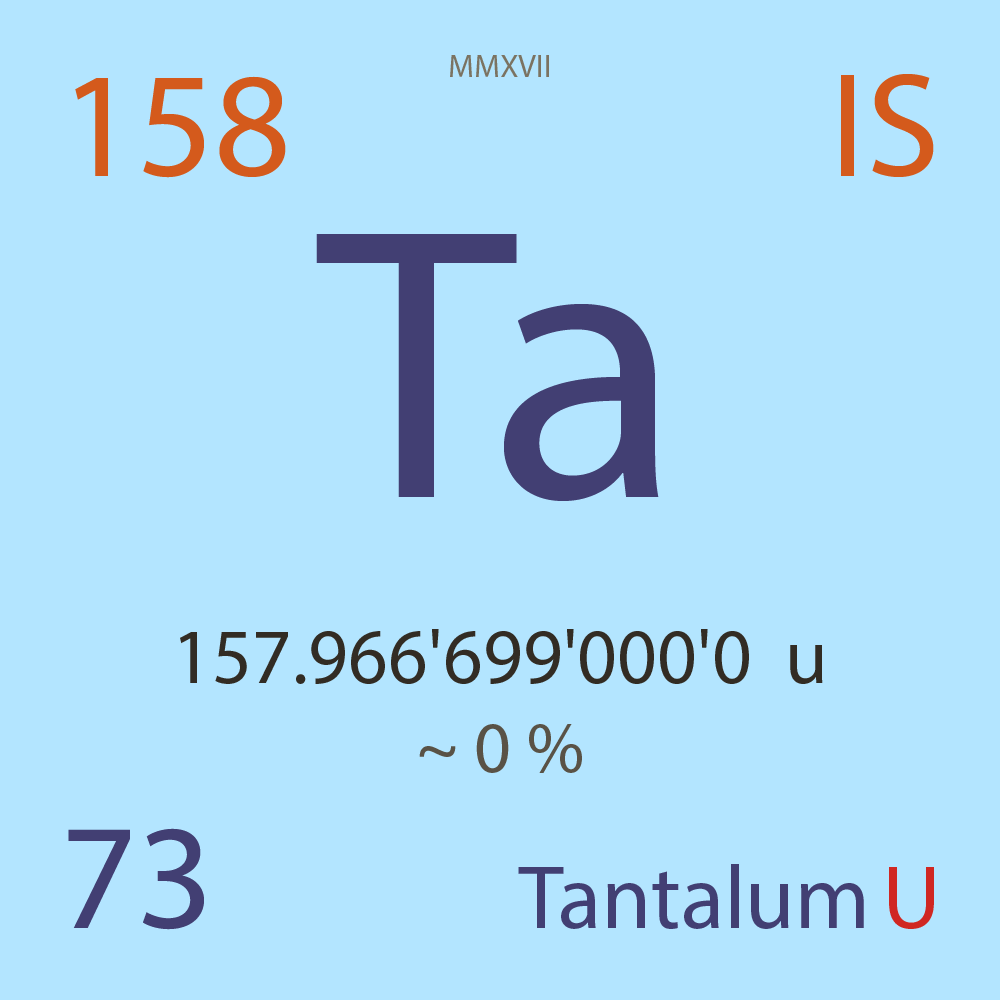
| Isotope_073_ta_158_u |
Unstable |
¹⁵⁸Ta |
Fermion |
73 |
p |
85 |
n |
2 |
-1 |
157.966'699'000'0 |
u |
~ 0 |
% |
~ 0 |
-31.019'000'000'0 |
MeV |
7.906'000'000'0 |
MeV |
- |
|
- |
|
1.55E-12 |
year |
49.000 |
micro-seconds ( x⁻⁶ ) |
96.000'000 |
% |
α |
6,123.500 |
keV |
¹⁵⁴Lu |
¹⁵⁸Ta > [ 96 % , α , 6,123.5 keV ] > ¹⁵⁴Lu |
|
|
β+ |
10,063.000 |
keV |
¹⁵⁸Hf |
¹⁵⁸Ta > [ , β+ , 10,063.0 keV ] > ¹⁵⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁴²Nd |
? |
% |
¹⁵⁴Gd |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁸Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_159_u |
Unstable |
¹⁵⁹Ta |
Boson |
73 |
p |
86 |
n |
1/2 |
1 |
158.963'018'173'0 |
u |
~ 0 |
% |
~ 0 |
-34.448'350'000'0 |
MeV |
7.928'783'000'0 |
MeV |
- |
|
- |
|
3.30E-8 |
year |
1.040 |
seconds ( x⁰ ) |
66.000'000 |
% |
β+ |
7,383.000 |
keV |
¹⁵⁹Hf |
¹⁵⁹Ta > [ 66 % , β+ , 7,383.0 keV ] > ¹⁵⁹Hf |
|
|
α |
5,680.910 |
keV |
¹⁵⁵Lu |
¹⁵⁹Ta > [ , α , 5,680.91 keV ] > ¹⁵⁵Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
42.900'000 |
% |
¹⁵⁹Tb |
37.205'373 |
% |
¹⁵¹Eu |
13.327'107 |
% |
¹⁴³Nd |
2.534'806 |
% |
¹⁵⁵Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
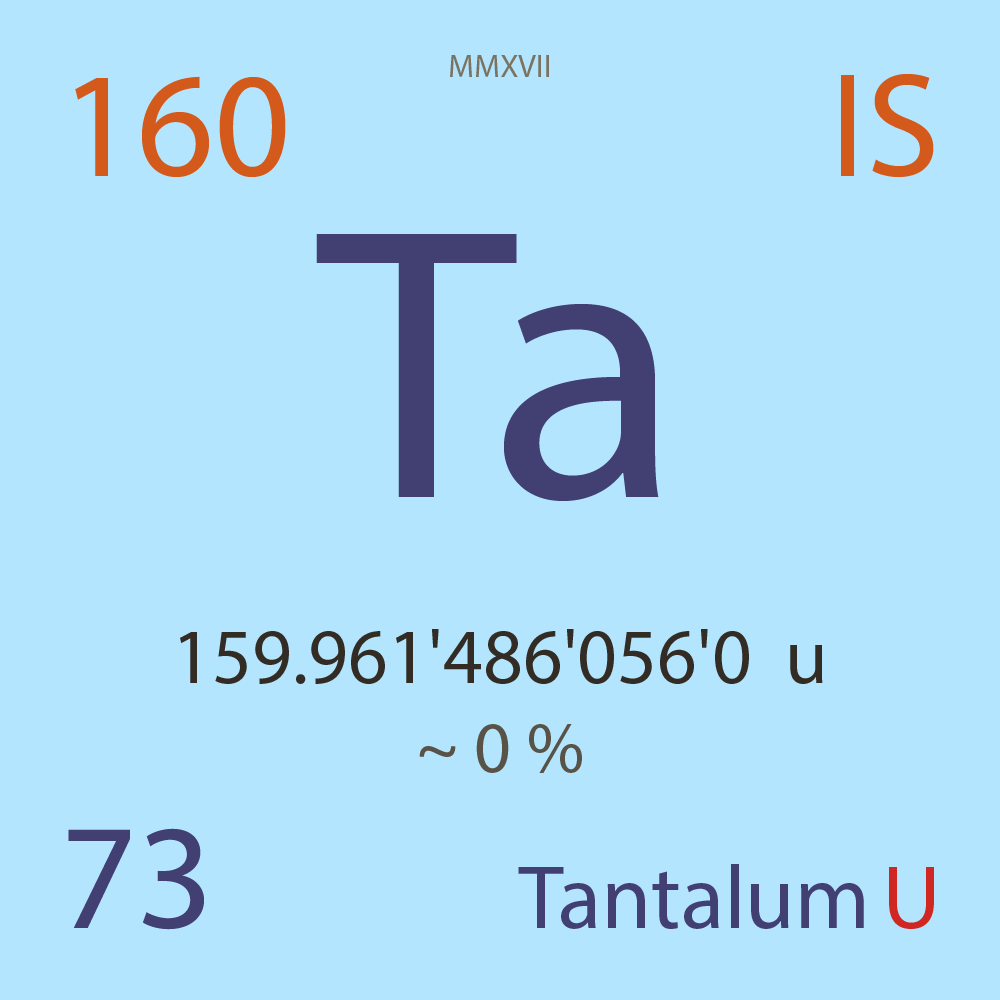
| Isotope_073_ta_160_u |
Unstable |
¹⁶⁰Ta |
Fermion |
73 |
p |
87 |
n |
2 |
-1 |
159.961'486'056'0 |
u |
~ 0 |
% |
~ 0 |
-35.875'507'000'0 |
MeV |
7.938'593'000'0 |
MeV |
- |
|
- |
|
5.39E-8 |
year |
1.700 |
seconds ( x⁰ ) |
? |
% |
β+ |
9,039.500 |
keV |
¹⁶⁰Hf |
¹⁶⁰Ta > [ ? % , β+ , 9,039.5 keV ] > ¹⁶⁰Hf |
|
|
α |
5,449.500 |
keV |
¹⁵⁶Lu |
¹⁶⁰Ta > [ , α , 5,449.5 keV ] > ¹⁵⁶Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁶⁰Dy |
? |
% |
¹⁴⁰Ce |
? |
% |
¹⁵²Sm |
? |
% |
¹⁵⁶Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
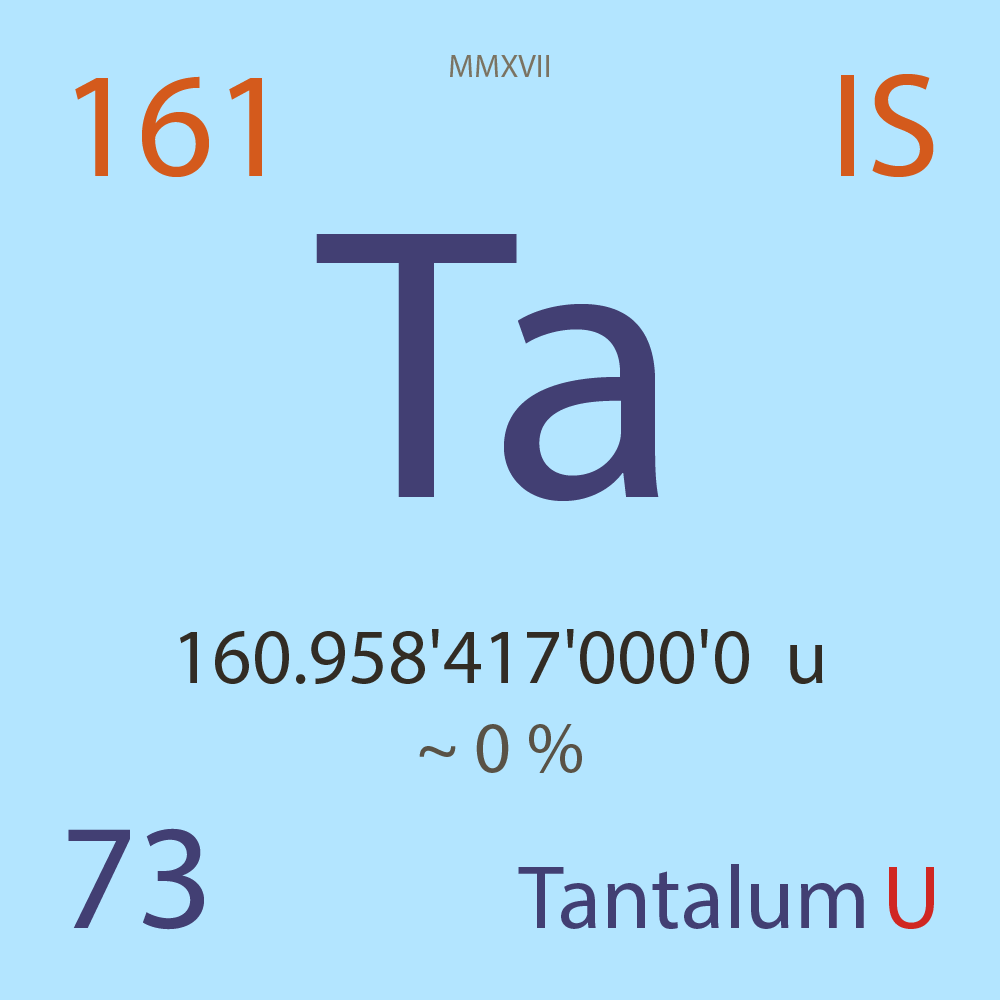
| Isotope_073_ta_161_u |
Unstable |
¹⁶¹Ta |
Boson |
73 |
p |
88 |
n |
1/2 |
1 |
160.958'417'000'0 |
u |
~ 0 |
% |
~ 0 |
-38.734'000'000'0 |
MeV |
7.957'000'000'0 |
MeV |
- |
|
- |
|
9.51E-8 |
year |
3.000 |
seconds ( x⁰ ) |
? |
% |
β+ |
6,562.800 |
keV |
¹⁶¹Hf |
¹⁶¹Ta > [ ? % , β+ , 6,562.8 keV ] > ¹⁶¹Hf |
|
|
α |
5,324.000 |
keV |
¹⁵⁷Lu |
¹⁶¹Ta > [ , α , 5,324.0 keV ] > ¹⁵⁷Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁴¹Pr |
? |
% |
¹⁴⁵Nd |
? |
% |
¹⁵⁷Gd |
? |
% |
¹⁵³Eu |
? |
% |
¹⁶¹Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_162_u |
Unstable |
¹⁶²Ta |
Fermion |
73 |
p |
89 |
n |
3 |
1 |
161.957'291'859'0 |
u |
~ 0 |
% |
~ 0 |
-39.782'377'000'0 |
MeV |
7.964'348'000'0 |
MeV |
- |
|
- |
|
1.13E-7 |
year |
3.570 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
8,368.500 |
keV |
¹⁶²Hf |
¹⁶²Ta > [ 100 % , β+ , 8,368.5 keV ] > ¹⁶²Hf |
|
|
α |
5,007.100 |
keV |
¹⁵⁸Lu |
¹⁶²Ta > [ , α , 5,007.1 keV ] > ¹⁵⁸Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.000'677 |
% |
¹⁴²Nd |
? |
% |
¹⁵⁴Gd |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁸Gd |
? |
% |
¹⁶²Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_163_u |
Unstable |
¹⁶³Ta |
Boson |
73 |
p |
90 |
n |
1/2 |
1 |
162.954'330'271'0 |
u |
~ 0 |
% |
~ 0 |
-42.541'078'000'0 |
MeV |
7.981'929'000'0 |
MeV |
- |
|
- |
|
3.36E-7 |
year |
10.600 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
5,723.000 |
keV |
¹⁶³Hf |
¹⁶³Ta > [ 100 % , β+ , 5,723.0 keV ] > ¹⁶³Hf |
|
|
α |
4,748.980 |
keV |
¹⁵⁹Lu |
¹⁶³Ta > [ , α , 4,748.98 keV ] > ¹⁵⁹Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶³Dy |
0.200'100 |
% |
¹⁵⁹Tb |
0.000'196 |
% |
¹⁵⁵Gd |
0.000'003 |
% |
¹⁵¹Eu |
0.000'001 |
% |
¹⁴³Nd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
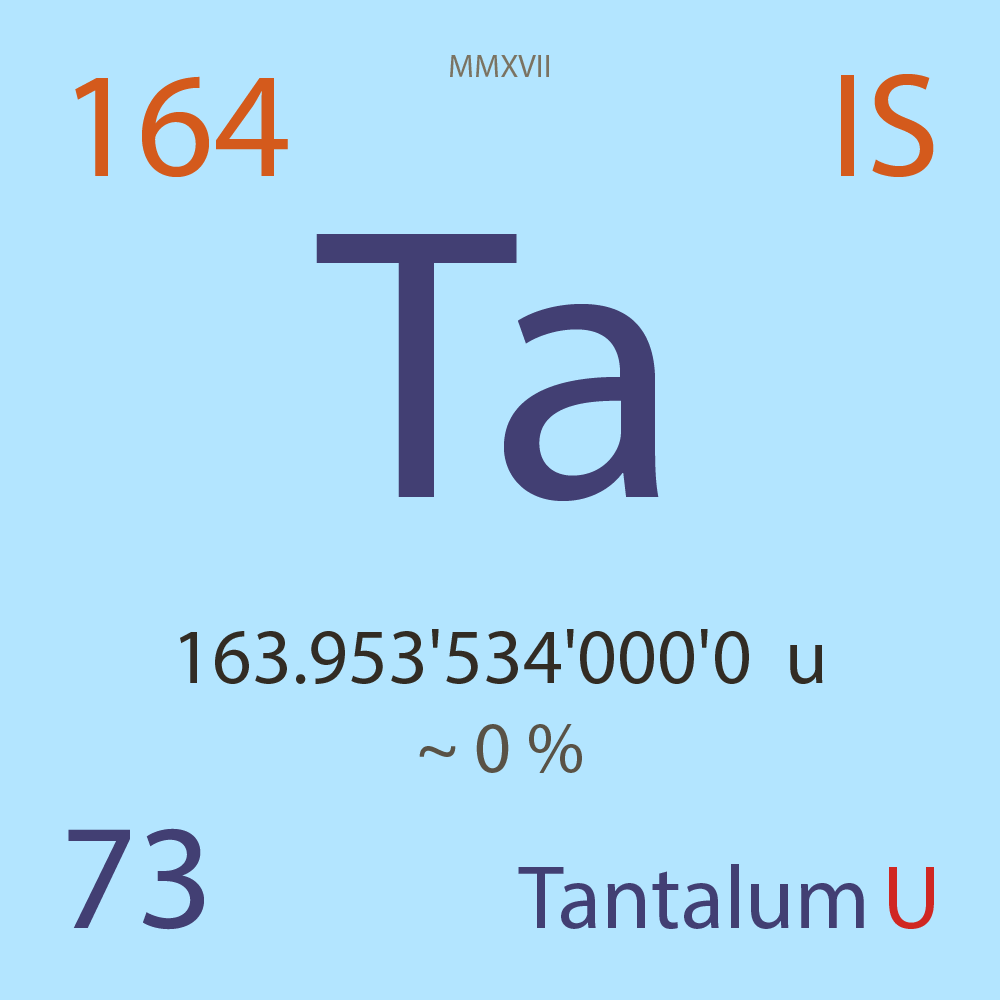
| Isotope_073_ta_164_u |
Unstable |
¹⁶⁴Ta |
Fermion |
73 |
p |
91 |
n |
3 |
1 |
163.953'534'000'0 |
u |
~ 0 |
% |
~ 0 |
-43.282'801'000'0 |
MeV |
7.986'997'000'0 |
MeV |
- |
|
- |
|
4.50E-7 |
year |
14.200 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
7,516.500 |
keV |
¹⁶⁴Hf |
¹⁶⁴Ta > [ 100 % , β+ , 7,516.5 keV ] > ¹⁶⁴Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁶⁴Dy |
? |
% |
¹⁶⁰Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_165_u |
Unstable |
¹⁶⁵Ta |
Boson |
73 |
p |
92 |
n |
5/2 |
-1 |
164.950'772'514'0 |
u |
~ 0 |
% |
~ 0 |
-45.855'107'000'0 |
MeV |
8.003'098'000'0 |
MeV |
- |
|
- |
|
9.83E-7 |
year |
31.020 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
4,758.200 |
keV |
¹⁶⁵Hf |
¹⁶⁵Ta > [ 100 % , β+ , 4,758.2 keV ] > ¹⁶⁵Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶⁵Ho |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
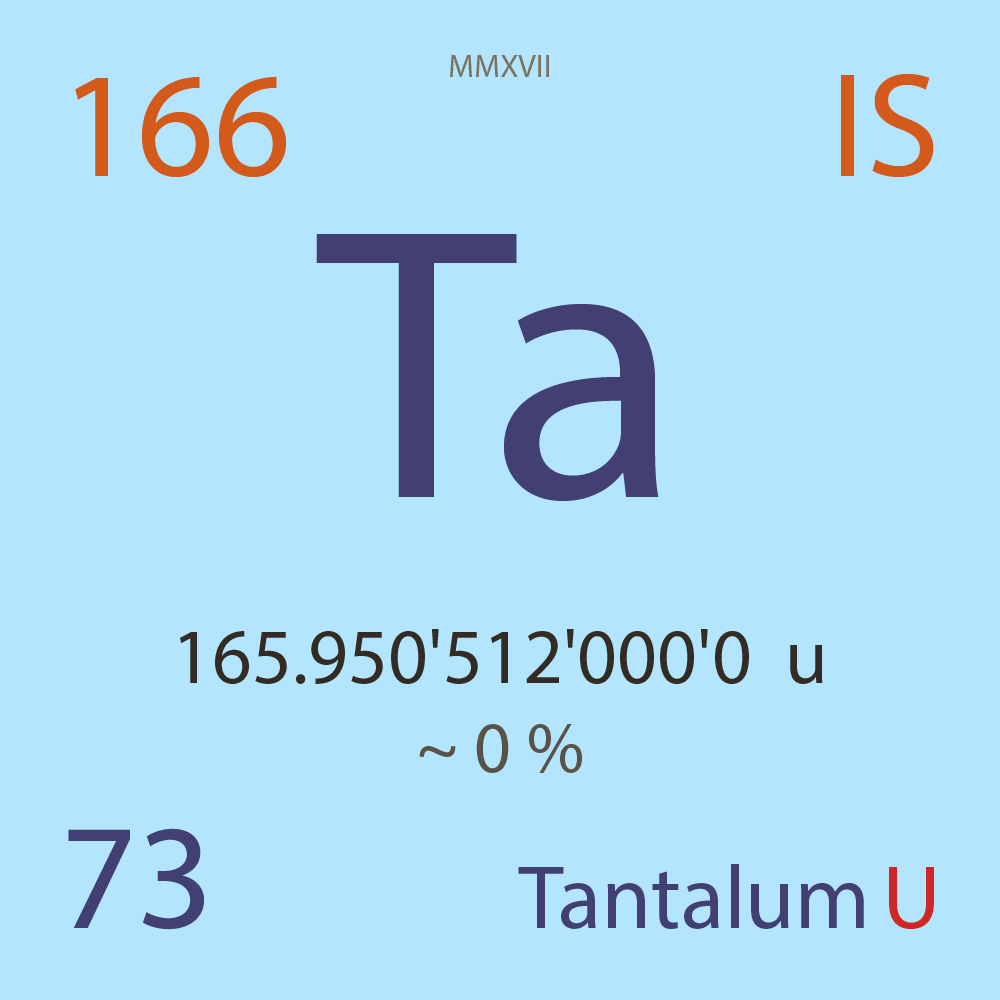
| Isotope_073_ta_166_u |
Unstable |
¹⁶⁶Ta |
Fermion |
73 |
p |
93 |
n |
2 |
1 |
165.950'512'000'0 |
u |
~ 0 |
% |
~ 0 |
-46.097'776'000'0 |
MeV |
8.004'971'000'0 |
MeV |
- |
|
- |
|
1.09E-6 |
year |
34.400 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
6,739.000 |
keV |
¹⁶⁶Hf |
¹⁶⁶Ta > [ 100 % , β+ , 6,739.0 keV ] > ¹⁶⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶⁶Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
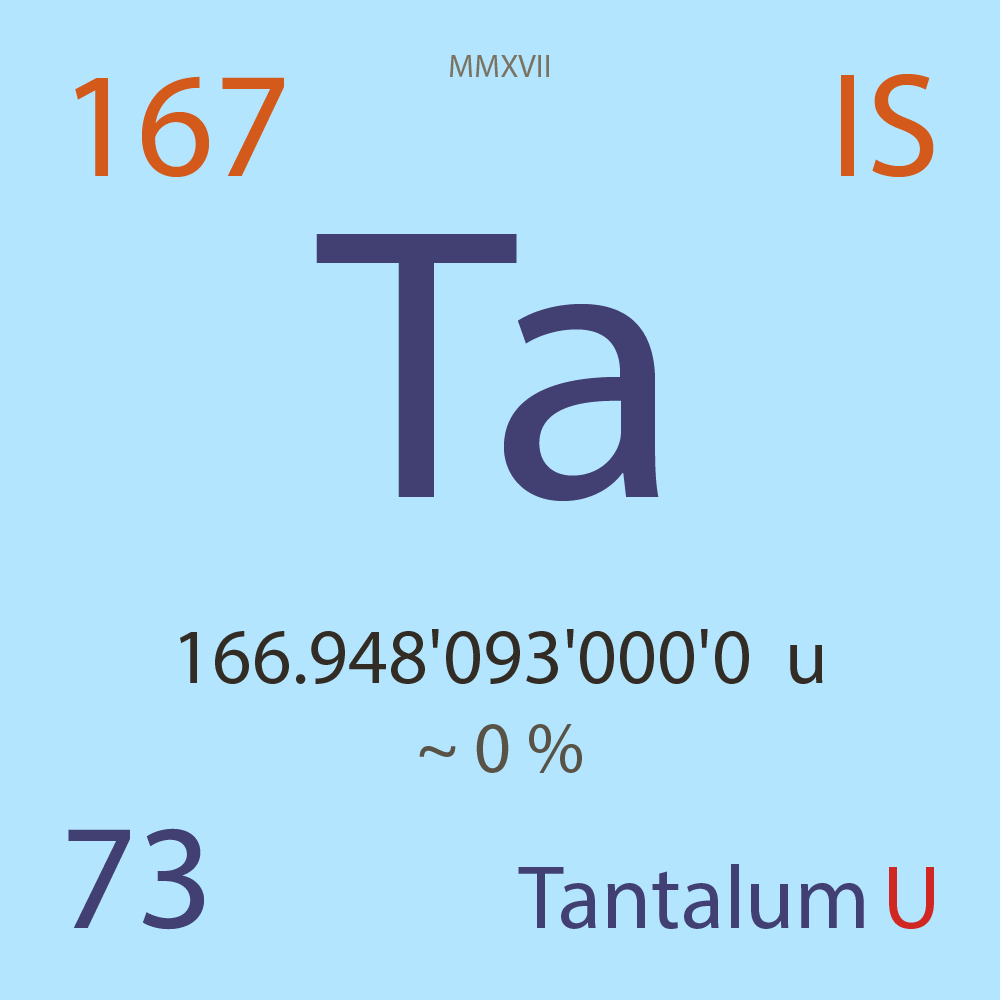
| Isotope_073_ta_167_u |
Unstable |
¹⁶⁷Ta |
Boson |
73 |
p |
94 |
n |
3/2 |
1 |
166.948'093'000'0 |
u |
~ 0 |
% |
~ 0 |
-48.351'060'000'0 |
MeV |
8.018'861'000'0 |
MeV |
- |
|
- |
|
2.53E-6 |
year |
79.800 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
4,094.500 |
keV |
¹⁶⁷Hf |
¹⁶⁷Ta > [ 100 % , β+ , 4,094.5 keV ] > ¹⁶⁷Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶⁷Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_168_u |
Unstable |
¹⁶⁸Ta |
Fermion |
73 |
p |
95 |
n |
? |
0 |
167.948'047'000'0 |
u |
~ 0 |
% |
~ 0 |
-48.393'908'000'0 |
MeV |
8.019'428'000'0 |
MeV |
- |
|
- |
|
3.80E-6 |
year |
120.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
5,944.400 |
keV |
¹⁶⁸Hf |
¹⁶⁸Ta > [ 100 % , β+ , 5,944.4 keV ] > ¹⁶⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁶⁸Er |
? |
% |
¹⁶⁴Dy |
? |
% |
¹⁶⁰Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
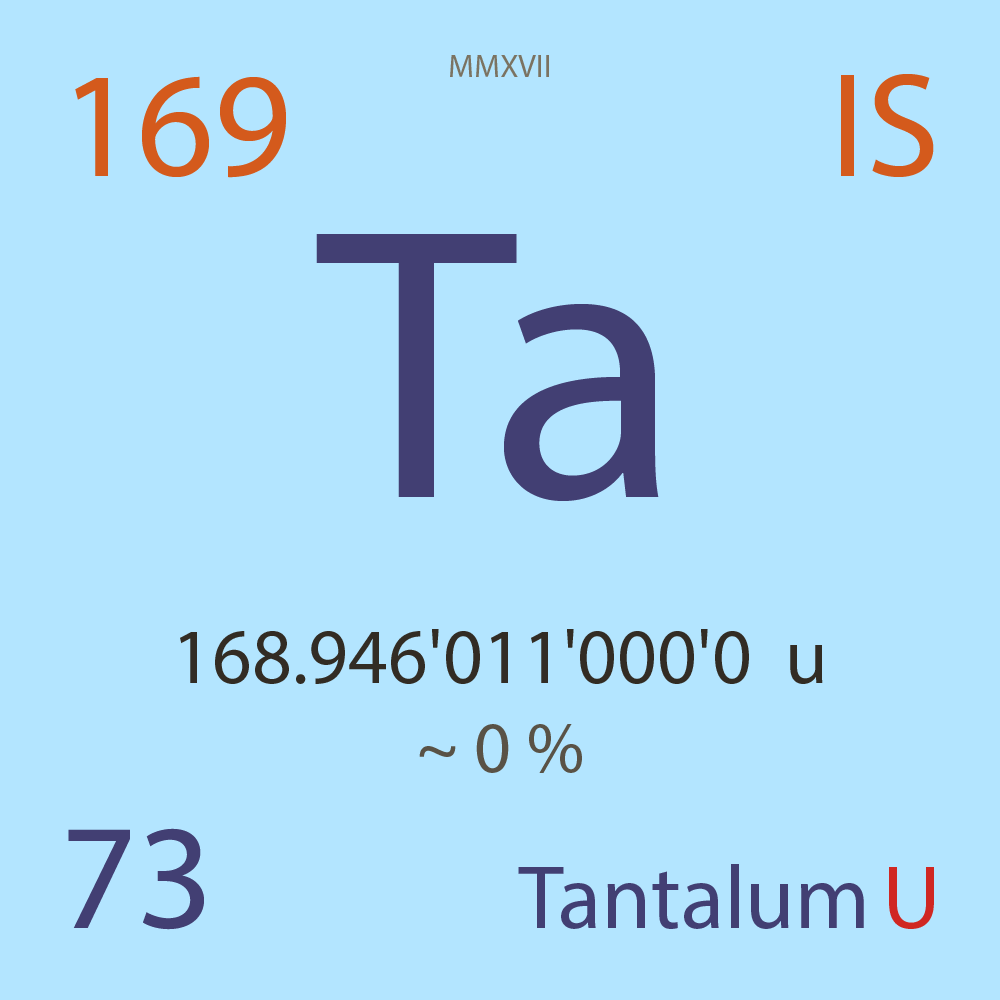
| Isotope_073_ta_169_u |
Unstable |
¹⁶⁹Ta |
Boson |
73 |
p |
96 |
n |
5/2 |
1 |
168.946'011'000'0 |
u |
~ 0 |
% |
~ 0 |
-50.290'430'000'0 |
MeV |
8.030'957'000'0 |
MeV |
- |
|
- |
|
1.53E-7 |
year |
4.833 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
3,404.300 |
keV |
¹⁶⁹Hf |
¹⁶⁹Ta > [ 100 % , β+ , 3,404.3 keV ] > ¹⁶⁹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶⁹Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_170_u |
Unstable |
¹⁷⁰Ta |
Fermion |
73 |
p |
97 |
n |
3 |
1 |
169.946'175'000'0 |
u |
~ 0 |
% |
~ 0 |
-50.137'665'000'0 |
MeV |
8.030'296'000'0 |
MeV |
- |
|
- |
|
2.14E-7 |
year |
6.767 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
5,094.000 |
keV |
¹⁷⁰Hf |
¹⁷⁰Ta > [ 100 % , β+ , 5,094.0 keV ] > ¹⁷⁰Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁰Yb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_171_u |
Unstable |
¹⁷¹Ta |
Boson |
73 |
p |
98 |
n |
5/2 |
-1 |
170.944'476'000'0 |
u |
~ 0 |
% |
~ 0 |
-51.720'273'000'0 |
MeV |
8.039'791'000'0 |
MeV |
- |
|
- |
|
7.38E-7 |
year |
23.300 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
2,688.900 |
keV |
¹⁷¹Hf |
¹⁷¹Ta > [ 100 % , β+ , 2,688.9 keV ] > ¹⁷¹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷¹Yb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_172_u |
Unstable |
¹⁷²Ta |
Fermion |
73 |
p |
99 |
n |
3 |
1 |
171.944'895'000'0 |
u |
~ 0 |
% |
~ 0 |
-51.329'977'000'0 |
MeV |
8.037'705'000'0 |
MeV |
- |
|
- |
|
1.17E-6 |
year |
36.833 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
4,051.400 |
keV |
¹⁷²Hf |
¹⁷²Ta > [ 100 % , β+ , 4,051.4 keV ] > ¹⁷²Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷²Yb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_173_u |
Unstable |
¹⁷³Ta |
Boson |
73 |
p |
100 |
n |
5/2 |
-1 |
172.943'750'000'0 |
u |
~ 0 |
% |
~ 0 |
-52.396'538'000'0 |
MeV |
8.044'064'000'0 |
MeV |
1.703'000'000'0 |
nm |
-1.900'000'000'0 |
b |
3.58E-4 |
year |
11.300 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
1,993.000 |
keV |
¹⁷³Hf |
¹⁷³Ta > [ 100 % , β+ , 1,993.0 keV ] > ¹⁷³Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷³Yb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_174_u |
Unstable |
¹⁷⁴Ta |
Fermion |
73 |
p |
101 |
n |
3 |
1 |
173.944'454'000'0 |
u |
~ 0 |
% |
~ 0 |
-51.740'766'000'0 |
MeV |
8.040'452'000'0 |
MeV |
- |
|
- |
|
1.30E-4 |
year |
4.104 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
3,083.700 |
keV |
¹⁷⁴Hf |
¹⁷⁴Ta > [ 100 % , β+ , 3,083.7 keV ] > ¹⁷⁴Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁰Yb |
? |
% |
¹⁷⁴Yb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_175_u |
Unstable |
¹⁷⁵Ta |
Boson |
73 |
p |
102 |
n |
7/2 |
1 |
174.943'737'000'0 |
u |
~ 0 |
% |
~ 0 |
-52.408'647'000'0 |
MeV |
8.044'445'000'0 |
MeV |
2.270'000'000'0 |
nm |
3.650'000'000'0 |
b |
1.20E-3 |
year |
37.800 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
1,053.000 |
keV |
¹⁷⁵Hf |
¹⁷⁵Ta > [ 100 % , β+ , 1,053.0 keV ] > ¹⁷⁵Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁵Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_176_u |
Unstable |
¹⁷⁶Ta |
Fermion |
73 |
p |
103 |
n |
1 |
-1 |
175.944'857'000'0 |
u |
~ 0 |
% |
~ 0 |
-51.365'374'000'0 |
MeV |
8.038'670'000'0 |
MeV |
- |
|
- |
|
9.22E-4 |
year |
29.099 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
2,189.900 |
keV |
¹⁷⁶Hf |
¹⁷⁶Ta > [ 100 % , β+ , 2,189.9 keV ] > ¹⁷⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_177_u |
Unstable |
¹⁷⁷Ta |
Boson |
73 |
p |
104 |
n |
7/2 |
1 |
176.944'472'403'0 |
u |
~ 0 |
% |
~ 0 |
-51.723'623'000'0 |
MeV |
8.040'878'000'0 |
MeV |
2.250'000'000'0 |
nm |
- |
|
6.45E-3 |
year |
203.602 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
143.800 |
keV |
¹⁷⁷Hf |
¹⁷⁷Ta > [ 100 % , β+ , 143.8 keV ] > ¹⁷⁷Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁷Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_178_u |
Unstable |
¹⁷⁸Ta |
Fermion |
73 |
p |
105 |
n |
1 |
1 |
177.945'778'221'0 |
u |
~ 0 |
% |
~ 0 |
-50.507'262'000'0 |
MeV |
8.034'216'000'0 |
MeV |
2.740'000'000'0 |
nm |
0.650'000'000'0 |
b |
1.77E-5 |
year |
559.020 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
914.800 |
keV |
¹⁷⁸Hf |
¹⁷⁸Ta > [ 100 % , β+ , 914.8 keV ] > ¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_179_u |
Unstable |
¹⁷⁹Ta |
Boson |
73 |
p |
106 |
n |
7/2 |
1 |
178.945'929'535'0 |
u |
~ 0 |
% |
~ 0 |
-50.366'314'000'0 |
MeV |
8.033'636'000'0 |
MeV |
100.000'000'000'0 |
nm |
- |
|
1.82E+0 |
years |
57.435 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
ϵ |
105.622 |
keV |
¹⁷⁹Hf |
¹⁷⁹Ta > [ 100 % , ϵ , 105.622 keV ] > ¹⁷⁹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_180_u |
Unstable |
¹⁸⁰Ta |
Fermion |
73 |
p |
107 |
n |
1 |
1 |
179.947'464'831'0 |
u |
0.012'000 |
% |
0.021'593'695'8 |
-48.936'195'000'0 |
MeV |
8.025'900'000'0 |
MeV |
- |
|
- |
|
9.30E-4 |
year |
29.350 |
kilo-seconds ( x³ ) |
86.000'000 |
% |
ϵ |
852.200 |
keV |
¹⁸⁰Hf |
¹⁸⁰Ta > [ 86 % , ϵ , 852.2 keV ] > ¹⁸⁰Hf |
|
|
β- |
708.280 |
keV |
¹⁸⁰W |
¹⁸⁰Ta > [ , β- , 708.28 keV ] > ¹⁸⁰W |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
86.000'000 |
% |
¹⁸⁰Hf |
? |
% |
¹⁷⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_181_s |
Stable |
¹⁸¹Ta |
Boson |
73 |
p |
108 |
n |
7/2 |
1 |
180.947'995'763'0 |
u |
99.988'000 |
% |
180.926'282'003'5 |
-48.441'634'000'0 |
MeV |
8.023'418'000'0 |
MeV |
2.370'500'000'0 |
nm |
3.280'000'000'0 |
b |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
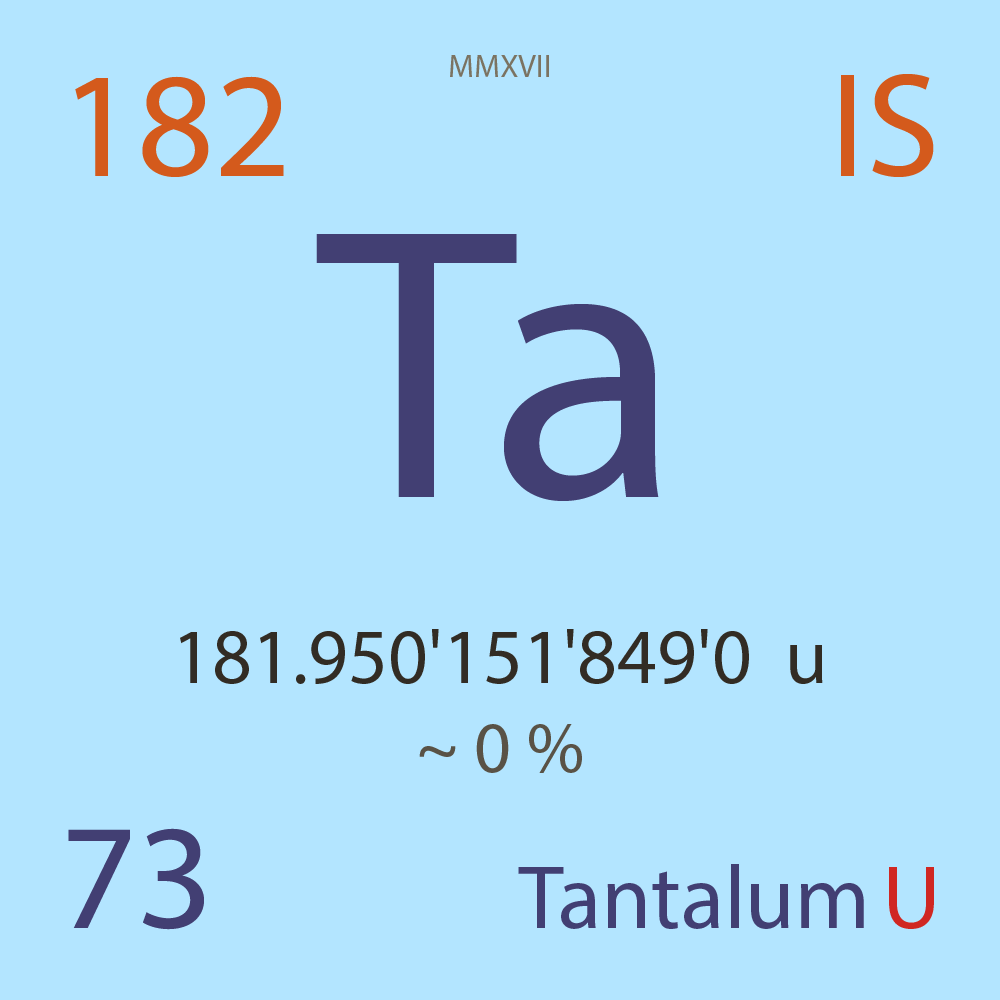
| Isotope_073_ta_182_u |
Unstable |
¹⁸²Ta |
Fermion |
73 |
p |
109 |
n |
3 |
-1 |
181.950'151'849'0 |
u |
~ 0 |
% |
~ 0 |
-46.433'254'000'0 |
MeV |
8.012'647'000'0 |
MeV |
3.020'000'000'0 |
nm |
209.000'000'000'0 |
b |
3.13E-1 |
year |
9.887 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
β- |
1,814.260 |
keV |
¹⁸²W |
¹⁸²Ta > [ 100 % , β- , 1,814.26 keV ] > ¹⁸²W |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
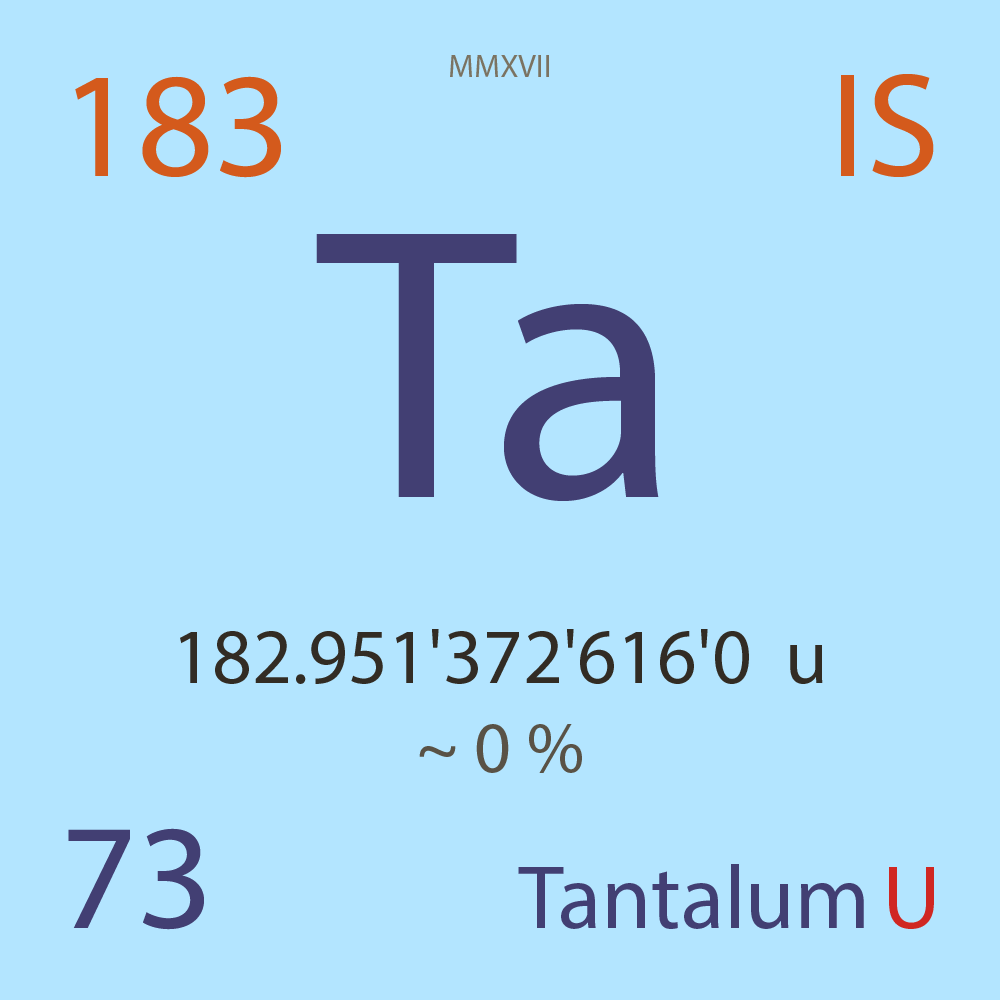
| Isotope_073_ta_183_u |
Unstable |
¹⁸³Ta |
Boson |
73 |
p |
110 |
n |
7/2 |
1 |
182.951'372'616'0 |
u |
~ 0 |
% |
~ 0 |
-45.296'117'000'0 |
MeV |
8.006'753'000'0 |
MeV |
2.360'000'000'0 |
nm |
- |
|
1.39E-2 |
year |
439.776 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
1,070.910 |
keV |
¹⁸³W |
¹⁸³Ta > [ 100 % , β- , 1,070.91 keV ] > ¹⁸³W |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁷⁹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_184_u |
Unstable |
¹⁸⁴Ta |
Fermion |
73 |
p |
111 |
n |
5 |
-1 |
183.954'007'966'0 |
u |
~ 0 |
% |
~ 0 |
-42.841'304'000'0 |
MeV |
7.993'763'000'0 |
MeV |
- |
|
- |
|
9.82E-4 |
year |
30.996 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
2,866.000 |
keV |
¹⁸⁴W |
¹⁸⁴Ta > [ 100 % , β- , 2,866.0 keV ] > ¹⁸⁴W |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁸⁰Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
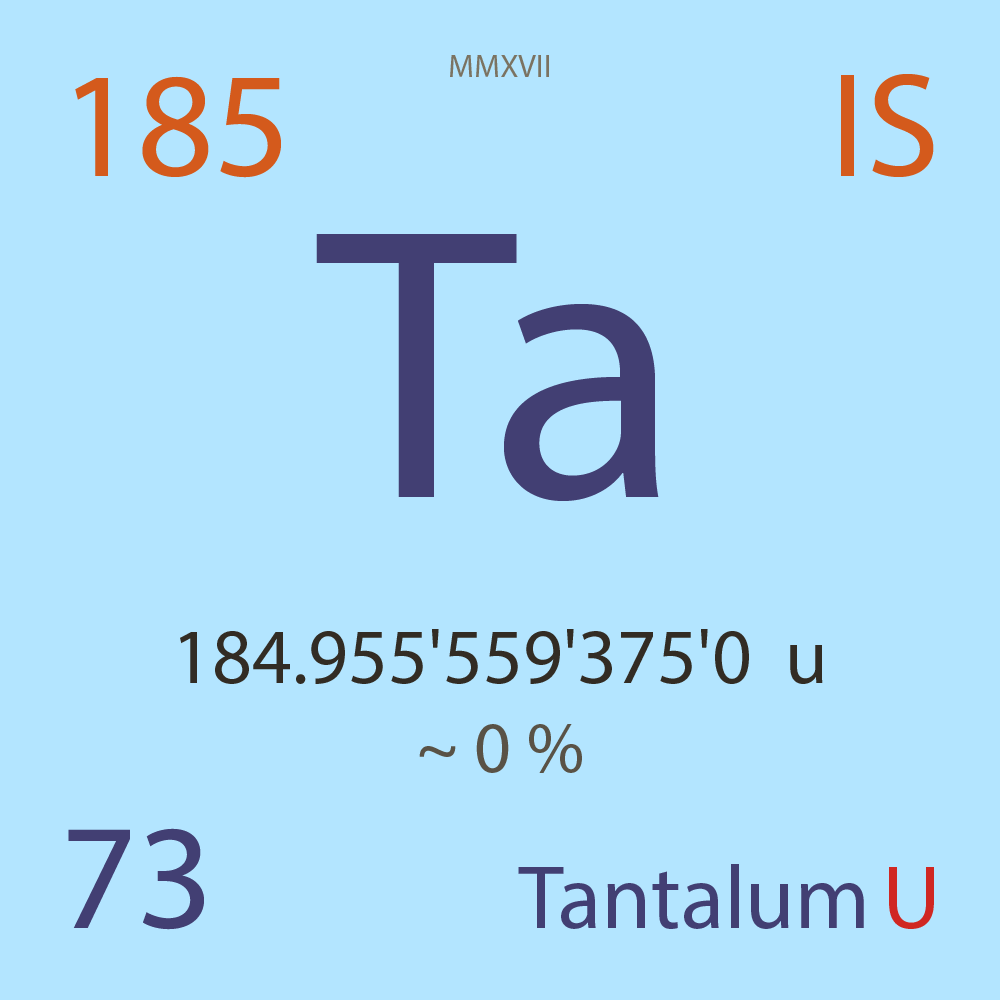
| Isotope_073_ta_185_u |
Unstable |
¹⁸⁵Ta |
Boson |
73 |
p |
112 |
n |
7/2 |
1 |
184.955'559'375'0 |
u |
~ 0 |
% |
~ 0 |
-41.396'176'000'0 |
MeV |
7.986'370'000'0 |
MeV |
- |
|
- |
|
9.38E-5 |
year |
2.960 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
1,993.500 |
keV |
¹⁸⁵W |
¹⁸⁵Ta > [ 100 % , β- , 1,993.5 keV ] > ¹⁸⁵W |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁸⁵Re |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_186_u |
Unstable |
¹⁸⁶Ta |
Fermion |
73 |
p |
113 |
n |
? |
0 |
185.958'552'023'0 |
u |
~ 0 |
% |
~ 0 |
-38.608'542'000'0 |
MeV |
7.971'840'000'0 |
MeV |
- |
|
- |
|
2.00E-5 |
year |
630.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
3,901.000 |
keV |
¹⁸⁶W |
¹⁸⁶Ta > [ 100 % , β- , 3,901.0 keV ] > ¹⁸⁶W |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
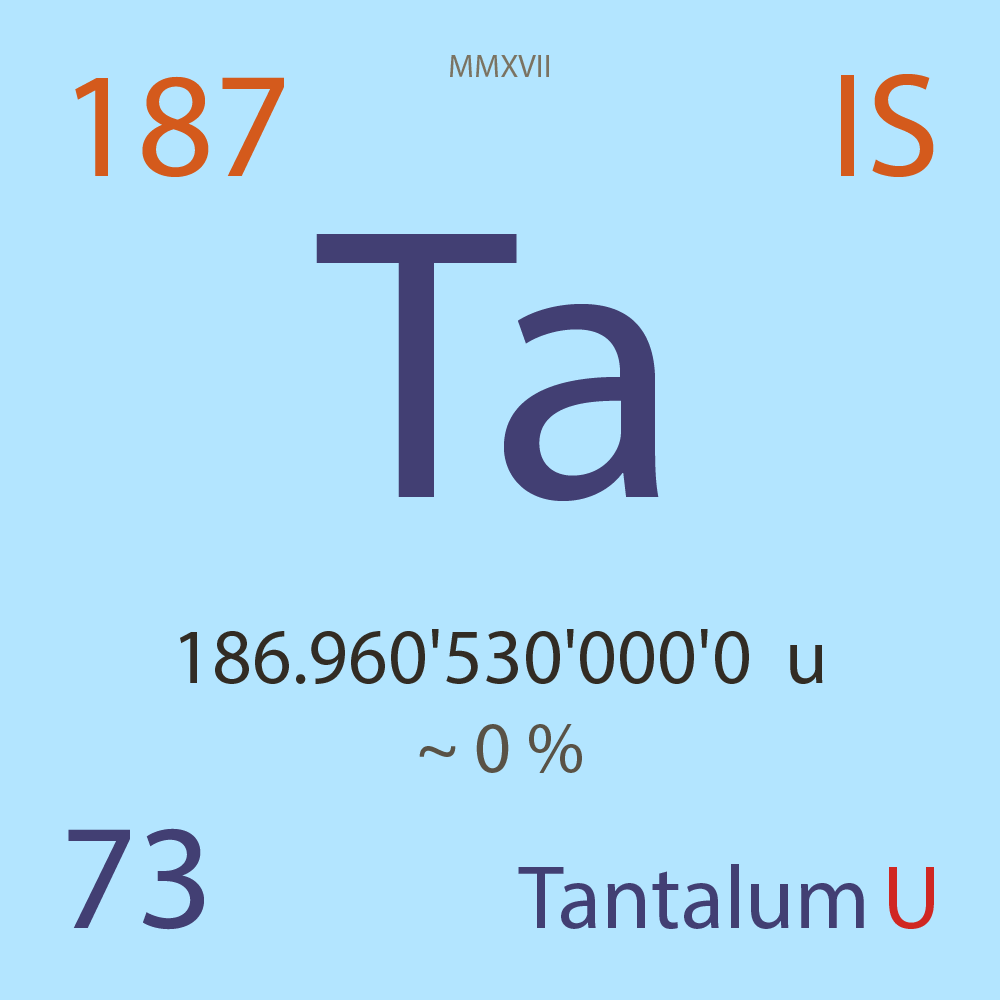
| Isotope_073_ta_187_u |
Unstable |
¹⁸⁷Ta |
Boson |
73 |
p |
114 |
n |
7/2 |
1 |
186.960'530'000'0 |
u |
~ 0 |
% |
~ 0 |
-36.766'000'000'0 |
MeV |
7.963'000'000'0 |
MeV |
- |
|
- |
|
3.18E-6 |
year |
100.200 |
seconds ( x⁰ ) |
? |
% |
β- |
3,139.000 |
keV |
¹⁸⁷W |
¹⁸⁷Ta > [ ? % , β- , 3,139.0 keV ] > ¹⁸⁷W |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁸⁷Os |
? |
% |
¹⁷⁹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
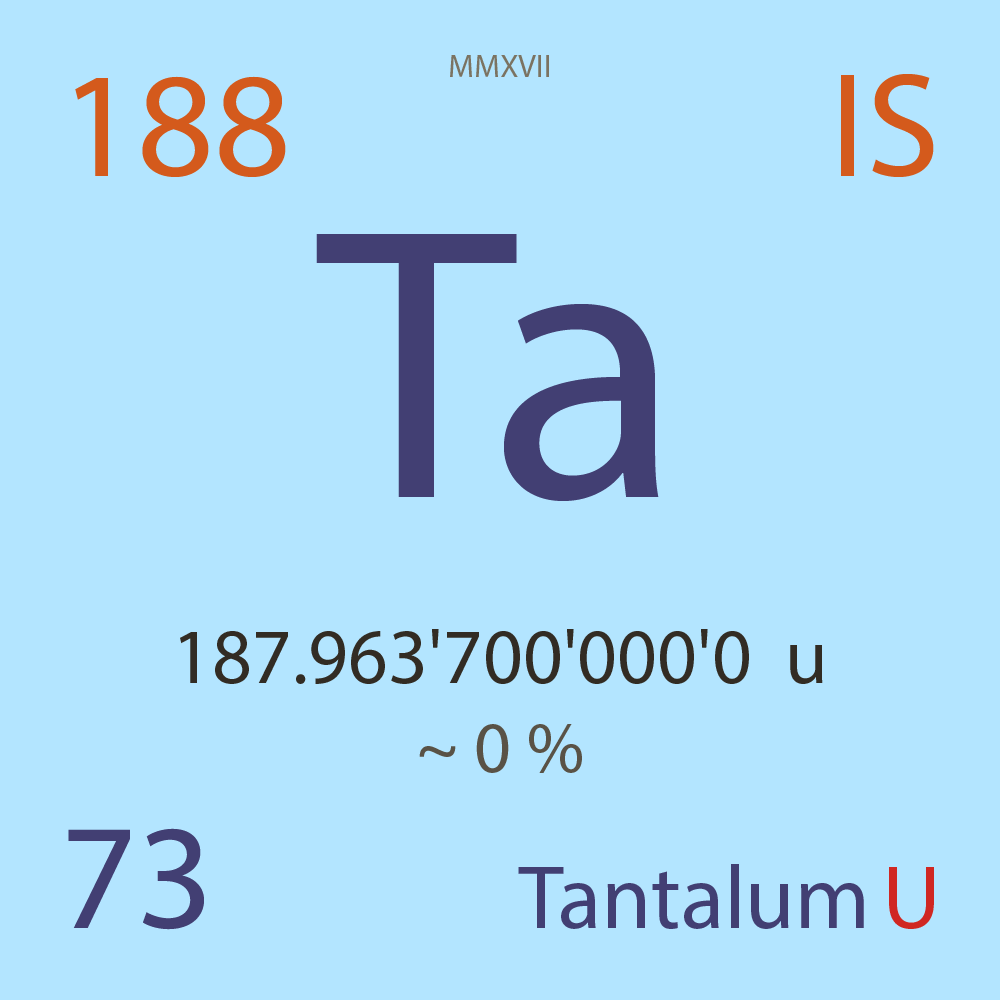
| Isotope_073_ta_188_u |
Unstable |
¹⁸⁸Ta |
Fermion |
73 |
p |
115 |
n |
? |
0 |
187.963'700'000'0 |
u |
~ 0 |
% |
~ 0 |
-33.813'000'000'0 |
MeV |
7.947'000'000'0 |
MeV |
- |
|
- |
|
6.34E-7 |
year |
20.000 |
seconds ( x⁰ ) |
? |
% |
β- |
4,854.000 |
keV |
¹⁸⁸W |
¹⁸⁸Ta > [ ? % , β- , 4,854.0 keV ] > ¹⁸⁸W |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁸⁸Os |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_073_ta_189_u |
Unstable |
¹⁸⁹Ta |
Boson |
73 |
p |
116 |
n |
7/2 |
1 |
188.965'830'000'0 |
u |
~ 0 |
% |
~ 0 |
-31.829'000'000'0 |
MeV |
7.938'000'000'0 |
MeV |
- |
|
- |
|
9.51E-8 |
year |
3.000 |
seconds ( x⁰ ) |
? |
% |
β- |
3,649.000 |
keV |
¹⁸⁹W |
¹⁸⁹Ta > [ ? % , β- , 3,649.0 keV ] > ¹⁸⁹W |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁸⁹Os |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
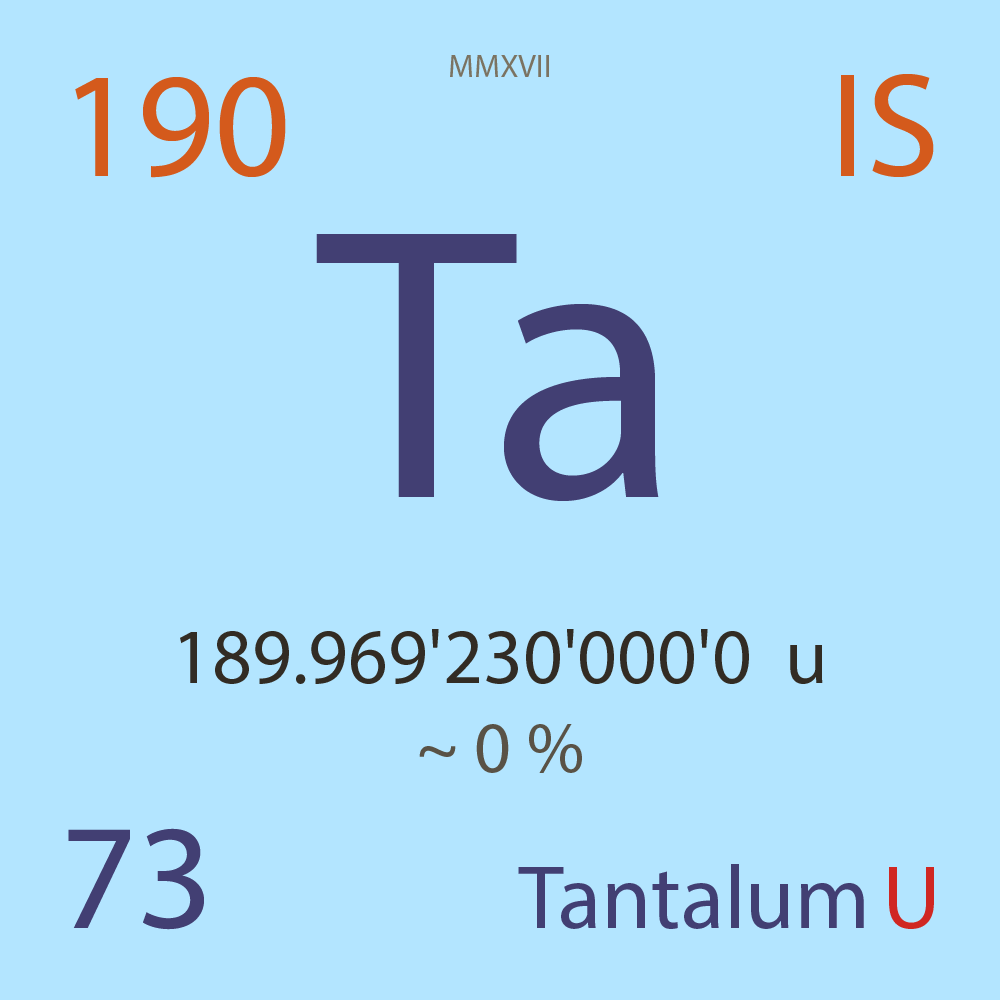
| Isotope_073_ta_190_u |
Unstable |
¹⁹⁰Ta |
Fermion |
73 |
p |
117 |
n |
? |
0 |
189.969'230'000'0 |
u |
~ 0 |
% |
~ 0 |
-28.662'000'000'0 |
MeV |
7.922'000'000'0 |
MeV |
- |
|
- |
|
9.51E-9 |
year |
300.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
5,634.000 |
keV |
¹⁹⁰W |
¹⁹⁰Ta > [ ? % , β- , 5,634.0 keV ] > ¹⁹⁰W |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁹⁰Os |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|