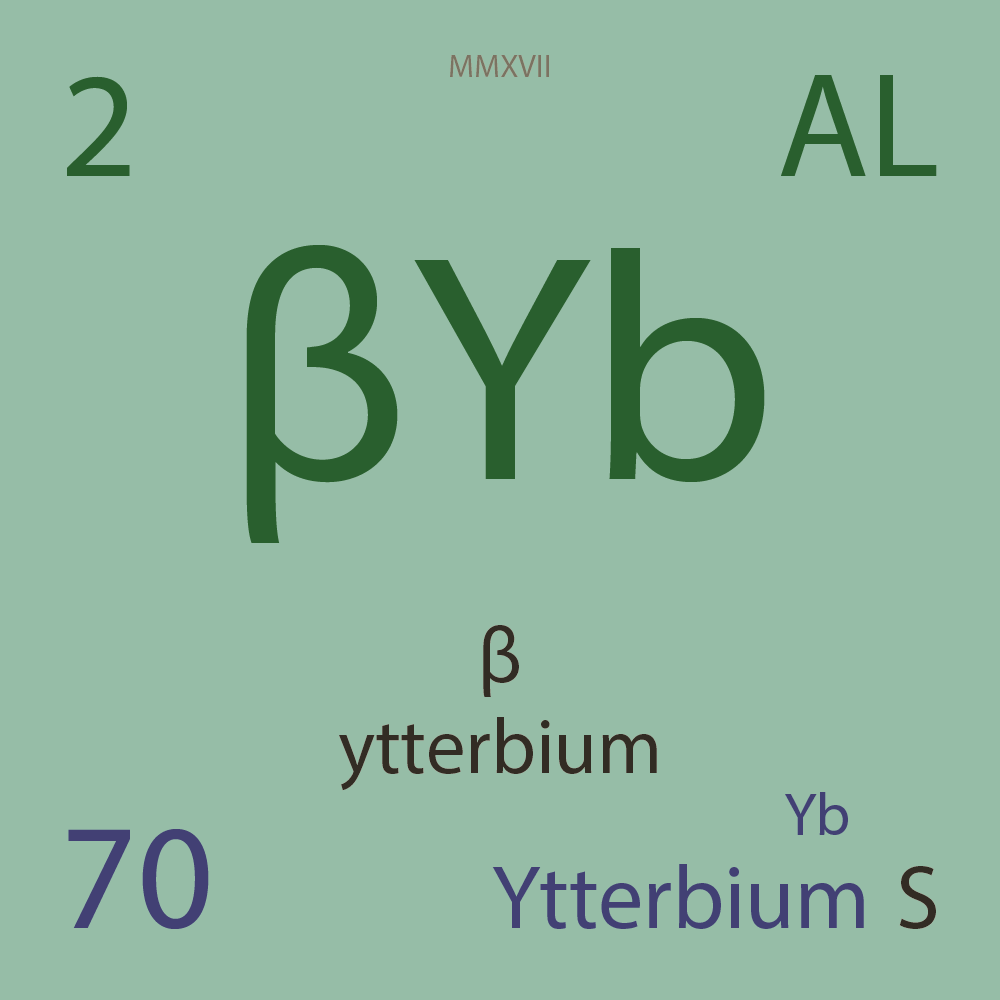
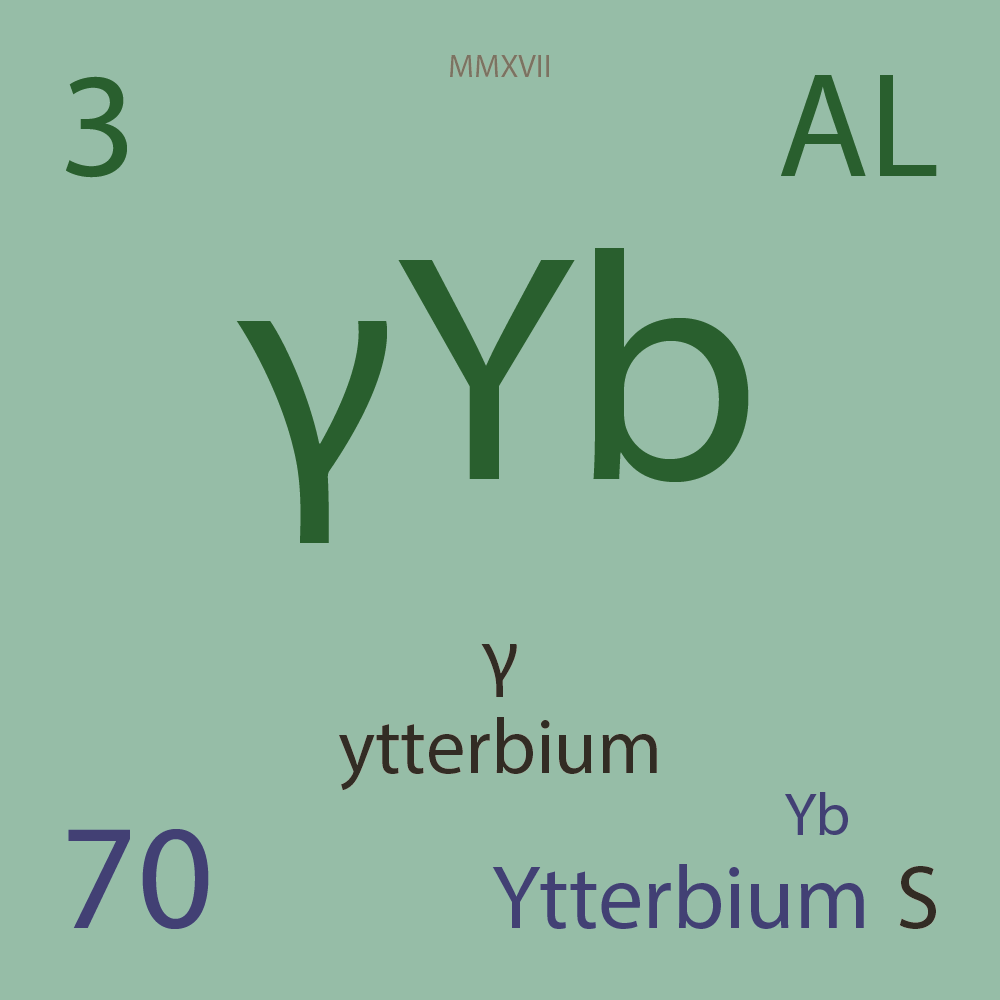
| Isotope_070_yb_148_u |
Unstable |
¹⁴⁸Yb |
Boson |
70 |
p |
78 |
n |
0 |
1 |
147.967'420'000'0 |
u |
~ 0 |
% |
~ 0 |
-30.348'000'000'0 |
MeV |
7.906'000'000'0 |
MeV |
- |
|
- |
|
7.92E-9 |
year |
250.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β+ |
7,902.000 |
keV |
¹⁴⁸Tm |
¹⁴⁸Yb > [ ? % , β+ , 7,902.0 keV ] > ¹⁴⁸Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁴³Nd |
? |
% |
¹⁴²Nd |
? |
% |
¹⁴⁰Ce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_149_u |
Unstable |
¹⁴⁹Yb |
Fermion |
70 |
p |
79 |
n |
? |
0 |
148.964'040'000'0 |
u |
~ 0 |
% |
~ 0 |
-33.497'000'000'0 |
MeV |
7.929'000'000'0 |
MeV |
- |
|
- |
|
2.22E-8 |
year |
700.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β+ |
9,523.000 |
keV |
¹⁴⁹Tm |
¹⁴⁹Yb > [ 100 % , β+ , 9,523.0 keV ] > ¹⁴⁹Tm |
|
|
β+p |
? |
keV |
¹⁴⁸Er |
¹⁴⁹Yb > [ , β+p , ? keV ] > ¹⁴⁸Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
15.531'333 |
% |
¹⁴⁵Nd |
0.000'000 |
% |
¹⁴¹Pr |
? |
% |
¹⁴³Nd |
? |
% |
¹⁴²Nd |
? |
% |
¹⁴⁰Ce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_150_u |
Unstable |
¹⁵⁰Yb |
Boson |
70 |
p |
80 |
n |
0 |
1 |
149.958'420'000'0 |
u |
~ 0 |
% |
~ 0 |
-38.732'000'000'0 |
MeV |
7.964'000'000'0 |
MeV |
- |
|
- |
|
2.22E-8 |
year |
700.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β+ |
6,858.000 |
keV |
¹⁵⁰Tm |
¹⁵⁰Yb > [ ? % , β+ , 6,858.0 keV ] > ¹⁵⁰Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁴²Nd |
? |
% |
¹⁵⁰Sm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_151_u |
Unstable |
¹⁵¹Yb |
Fermion |
70 |
p |
81 |
n |
1/2 |
1 |
150.955'400'769'0 |
u |
~ 0 |
% |
~ 0 |
-41.543'916'000'0 |
MeV |
7.983'765'000'0 |
MeV |
- |
|
- |
|
5.07E-8 |
year |
1.600 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
8,216.000 |
keV |
¹⁵¹Tm |
¹⁵¹Yb > [ 100 % , β+ , 8,216.0 keV ] > ¹⁵¹Tm |
|
|
β+p |
? |
keV |
¹⁵⁰Er |
¹⁵¹Yb > [ , β+p , ? keV ] > ¹⁵⁰Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
73.632'000 |
% |
¹⁵¹Eu |
26.375'576 |
% |
¹⁴³Nd |
? |
% |
¹⁴²Nd |
? |
% |
¹⁵⁰Sm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_152_u |
Unstable |
¹⁵²Yb |
Boson |
70 |
p |
82 |
n |
0 |
1 |
151.950'288'919'0 |
u |
~ 0 |
% |
~ 0 |
-46.305'574'000'0 |
MeV |
8.015'668'000'0 |
MeV |
- |
|
- |
|
9.63E-8 |
year |
3.040 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
4,443.000 |
keV |
¹⁵²Tm |
¹⁵²Yb > [ 100 % , β+ , 4,443.0 keV ] > ¹⁵²Tm |
|
|
β+p |
? |
keV |
¹⁵¹Er |
¹⁵²Yb > [ , β+p , ? keV ] > ¹⁵¹Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8.800'000 |
% |
¹⁴⁰Ce |
? |
% |
¹⁴³Nd |
? |
% |
¹⁵¹Eu |
? |
% |
¹⁵²Sm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_153_u |
Unstable |
¹⁵³Yb |
Fermion |
70 |
p |
83 |
n |
7/2 |
-1 |
152.949'480'000'0 |
u |
~ 0 |
% |
~ 0 |
-47.059'000'000'0 |
MeV |
8.021'000'000'0 |
MeV |
- |
|
- |
|
1.33E-7 |
year |
4.200 |
seconds ( x⁰ ) |
50.000'000 |
% |
α |
4,258.000 |
keV |
¹⁴⁹Er |
¹⁵³Yb > [ 50 % , α , 4,258.0 keV ] > ¹⁴⁹Er |
|
|
β+p |
? |
keV |
¹⁵²Er |
¹⁵³Yb > [ , β+p , ? keV ] > ¹⁵²Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7.765'667 |
% |
¹⁴⁵Nd |
0.000'704 |
% |
¹⁴⁰Ce |
0.000'000 |
% |
¹⁴¹Pr |
? |
% |
¹⁵²Sm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_154_u |
Unstable |
¹⁵⁴Yb |
Boson |
70 |
p |
84 |
n |
0 |
1 |
153.946'393'928'0 |
u |
~ 0 |
% |
~ 0 |
-49.933'735'000'0 |
MeV |
8.039'950'000'0 |
MeV |
- |
|
- |
|
1.30E-8 |
year |
409.000 |
milli-seconds ( x⁻³ ) |
92.600'000 |
% |
α |
5,474.240 |
keV |
¹⁵⁰Er |
¹⁵⁴Yb > [ 92.6 % , α , 5,474.24 keV ] > ¹⁵⁰Er |
|
|
β+ |
3,473.300 |
keV |
¹⁵⁴Tm |
¹⁵⁴Yb > [ , β+ , 3,473.3 keV ] > ¹⁵⁴Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.047'562 |
% |
¹⁴²Nd |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁴Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_155_u |
Unstable |
¹⁵⁵Yb |
Fermion |
70 |
p |
85 |
n |
7/2 |
-1 |
154.945'782'332'0 |
u |
~ 0 |
% |
~ 0 |
-50.503'433'000'0 |
MeV |
8.043'828'000'0 |
MeV |
-0.840'000'000'0 |
nm |
-1.200'000'000'0 |
b |
5.68E-8 |
year |
1.793 |
seconds ( x⁰ ) |
89.000'000 |
% |
α |
5,337.620 |
keV |
¹⁵¹Er |
¹⁵⁵Yb > [ 89 % , α , 5,337.62 keV ] > ¹⁵¹Er |
|
|
β+ |
5,109.700 |
keV |
¹⁵⁵Tm |
¹⁵⁵Yb > [ , β+ , 5,109.7 keV ] > ¹⁵⁵Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
65.688'612 |
% |
¹⁵¹Eu |
23.529'521 |
% |
¹⁴³Nd |
10.791'000 |
% |
¹⁵⁵Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_156_u |
Unstable |
¹⁵⁶Yb |
Boson |
70 |
p |
86 |
n |
0 |
1 |
155.942'818'215'0 |
u |
~ 0 |
% |
~ 0 |
-53.264'490'000'0 |
MeV |
8.061'703'000'0 |
MeV |
- |
|
- |
|
8.27E-7 |
year |
26.100 |
seconds ( x⁰ ) |
90.000'000 |
% |
β+ |
2,553.100 |
keV |
¹⁵⁶Tm |
¹⁵⁶Yb > [ 90 % , β+ , 2,553.1 keV ] > ¹⁵⁶Tm |
|
|
α |
4,810.770 |
keV |
¹⁵²Er |
¹⁵⁶Yb > [ , α , 4,810.77 keV ] > ¹⁵²Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.930'703 |
% |
¹⁴⁰Ce |
? |
% |
¹⁵²Sm |
? |
% |
¹⁵⁶Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_157_u |
Unstable |
¹⁵⁷Yb |
Fermion |
70 |
p |
87 |
n |
7/2 |
-1 |
156.942'627'848'0 |
u |
~ 0 |
% |
~ 0 |
-53.441'815'000'0 |
MeV |
8.062'894'000'0 |
MeV |
-0.639'000'000'0 |
nm |
- |
|
1.22E-6 |
year |
38.600 |
seconds ( x⁰ ) |
99.500'000 |
% |
β+ |
4,245.300 |
keV |
¹⁵⁷Tm |
¹⁵⁷Yb > [ 99.5 % , β+ , 4,245.3 keV ] > ¹⁵⁷Tm |
|
|
α |
4,621.240 |
keV |
¹⁵³Er |
¹⁵⁷Yb > [ , α , 4,621.24 keV ] > ¹⁵³Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
99.500'000 |
% |
¹⁵⁷Gd |
0.235'000 |
% |
¹⁵³Eu |
0.044'276 |
% |
¹⁴⁵Nd |
0.000'000 |
% |
¹⁴¹Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_158_u |
Unstable |
¹⁵⁸Yb |
Boson |
70 |
p |
88 |
n |
0 |
1 |
157.939'865'617'0 |
u |
~ 0 |
% |
~ 0 |
-56.014'817'000'0 |
MeV |
8.079'232'000'0 |
MeV |
- |
|
- |
|
2.83E-6 |
year |
89.400 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
1,666.200 |
keV |
¹⁵⁸Tm |
¹⁵⁸Yb > [ 100 % , β+ , 1,666.2 keV ] > ¹⁵⁸Tm |
|
|
α |
4,172.420 |
keV |
¹⁵⁴Er |
¹⁵⁸Yb > [ , α , 4,172.42 keV ] > ¹⁵⁴Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.002'110 |
% |
¹⁴²Nd |
? |
% |
¹⁵⁴Gd |
? |
% |
¹⁵⁰Sm |
? |
% |
¹⁵⁸Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_159_u |
Unstable |
¹⁵⁹Yb |
Fermion |
70 |
p |
89 |
n |
5/2 |
-1 |
158.940'050'099'0 |
u |
~ 0 |
% |
~ 0 |
-55.842'973'000'0 |
MeV |
8.078'101'000'0 |
MeV |
-0.366'000'000'0 |
nm |
-0.220'000'000'0 |
b |
3.26E-6 |
year |
103.020 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
3,705.200 |
keV |
¹⁵⁹Tm |
¹⁵⁹Yb > [ 100 % , β+ , 3,705.2 keV ] > ¹⁵⁹Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁵⁹Tb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_160_u |
Unstable |
¹⁶⁰Yb |
Boson |
70 |
p |
90 |
n |
0 |
1 |
159.937'552'344'0 |
u |
~ 0 |
% |
~ 0 |
-58.169'617'000'0 |
MeV |
8.092'601'000'0 |
MeV |
- |
|
- |
|
9.19E-6 |
year |
289.980 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
1,110.500 |
keV |
¹⁶⁰Tm |
¹⁶⁰Yb > [ 100 % , β+ , 1,110.5 keV ] > ¹⁶⁰Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶⁰Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_161_u |
Unstable |
¹⁶¹Yb |
Fermion |
70 |
p |
91 |
n |
3/2 |
-1 |
160.937'901'678'0 |
u |
~ 0 |
% |
~ 0 |
-57.844'214'000'0 |
MeV |
8.090'447'000'0 |
MeV |
-0.327'000'000'0 |
nm |
1.030'000'000'0 |
b |
7.92E-6 |
year |
250.020 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
3,032.300 |
keV |
¹⁶¹Tm |
¹⁶¹Yb > [ 100 % , β+ , 3,032.3 keV ] > ¹⁶¹Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶¹Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_162_u |
Unstable |
¹⁶²Yb |
Boson |
70 |
p |
92 |
n |
0 |
1 |
161.935'768'210'0 |
u |
~ 0 |
% |
~ 0 |
-59.831'527'000'0 |
MeV |
8.102'597'000'0 |
MeV |
- |
|
- |
|
3.59E-5 |
year |
1.132 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
629.800 |
keV |
¹⁶²Tm |
¹⁶²Yb > [ 100 % , β+ , 629.8 keV ] > ¹⁶²Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁵⁸Gd |
? |
% |
¹⁵⁴Gd |
? |
% |
¹⁶²Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_163_u |
Unstable |
¹⁶³Yb |
Fermion |
70 |
p |
93 |
n |
3/2 |
-1 |
162.936'334'305'0 |
u |
~ 0 |
% |
~ 0 |
-59.304'213'000'0 |
MeV |
8.099'170'000'0 |
MeV |
-0.374'000'000'0 |
nm |
1.240'000'000'0 |
b |
2.10E-5 |
year |
663.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
2,408.700 |
keV |
¹⁶³Tm |
¹⁶³Yb > [ 100 % , β+ , 2,408.7 keV ] > ¹⁶³Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶³Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_164_u |
Unstable |
¹⁶⁴Yb |
Boson |
70 |
p |
94 |
n |
0 |
1 |
163.934'489'416'0 |
u |
~ 0 |
% |
~ 0 |
-61.022'716'000'0 |
MeV |
8.109'478'000'0 |
MeV |
- |
|
- |
|
1.44E-4 |
year |
4.550 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
ϵ |
865.700 |
keV |
¹⁶⁴Tm |
¹⁶⁴Yb > [ 100 % , ϵ , 865.7 keV ] > ¹⁶⁴Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁶⁴Dy |
? |
% |
¹⁶⁰Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_165_u |
Unstable |
¹⁶⁵Yb |
Fermion |
70 |
p |
95 |
n |
5/2 |
-1 |
164.935'279'000'0 |
u |
~ 0 |
% |
~ 0 |
-60.287'224'000'0 |
MeV |
8.104'790'000'0 |
MeV |
0.478'000'000'0 |
nm |
2.480'000'000'0 |
b |
1.87E-5 |
year |
589.980 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
1,626.500 |
keV |
¹⁶⁵Tm |
¹⁶⁵Yb > [ 100 % , β+ , 1,626.5 keV ] > ¹⁶⁵Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶⁵Ho |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_166_u |
Unstable |
¹⁶⁶Yb |
Boson |
70 |
p |
96 |
n |
0 |
1 |
165.933'882'042'0 |
u |
~ 0 |
% |
~ 0 |
-61.588'481'000'0 |
MeV |
8.112'427'000'0 |
MeV |
- |
|
- |
|
6.46E-3 |
year |
203.990 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
ϵ |
305.400 |
keV |
¹⁶⁶Tm |
¹⁶⁶Yb > [ 100 % , ϵ , 305.4 keV ] > ¹⁶⁶Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶⁶Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_167_u |
Unstable |
¹⁶⁷Yb |
Fermion |
70 |
p |
97 |
n |
5/2 |
-1 |
166.934'949'605'0 |
u |
~ 0 |
% |
~ 0 |
-60.594'053'000'0 |
MeV |
8.106'226'000'0 |
MeV |
0.623'000'000'0 |
nm |
2.700'000'000'0 |
b |
3.33E-5 |
year |
1.050 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
932.060 |
keV |
¹⁶⁷Tm |
¹⁶⁷Yb > [ 100 % , β+ , 932.06 keV ] > ¹⁶⁷Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶⁷Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_168_s |
Stable |
¹⁶⁸Yb |
Boson |
70 |
p |
98 |
n |
0 |
1 |
167.933'896'895'0 |
u |
0.130'000 |
% |
0.218'314'066'0 |
-61.574'646'000'0 |
MeV |
8.111'855'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
? |
% |
α |
1,950.000 |
keV |
¹⁶⁴Er |
¹⁶⁸Yb > [ ? % , α , 1,950.0 keV ] > ¹⁶⁴Er |
|
|
2β+ |
-622.310 |
keV |
¹⁶⁸Er |
¹⁶⁸Yb > [ , 2β+ , -622.31 keV ] > ¹⁶⁸Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁶⁸Er |
? |
% |
¹⁶⁴Dy |
? |
% |
¹⁶⁰Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_169_u |
Unstable |
¹⁶⁹Yb |
Fermion |
70 |
p |
99 |
n |
7/2 |
1 |
168.935'189'802'0 |
u |
~ 0 |
% |
~ 0 |
-60.370'311'000'0 |
MeV |
8.104'489'000'0 |
MeV |
-0.635'000'000'0 |
nm |
3.540'000'000'0 |
b |
8.77E-2 |
year |
2.767 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
ϵ |
909.650 |
keV |
¹⁶⁹Tm |
¹⁶⁹Yb > [ 100 % , ϵ , 909.65 keV ] > ¹⁶⁹Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁶⁹Tm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_170_s |
Stable |
¹⁷⁰Yb |
Boson |
70 |
p |
100 |
n |
0 |
1 |
169.934'761'837'0 |
u |
3.040'000 |
% |
5.166'016'759'8 |
-60.768'957'000'0 |
MeV |
8.106'639'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_171_s |
Stable |
¹⁷¹Yb |
Fermion |
70 |
p |
101 |
n |
1/2 |
-1 |
170.936'325'799'0 |
u |
14.280'000 |
% |
24.409'707'324'1 |
-59.312'137'000'0 |
MeV |
8.097'913'000'0 |
MeV |
0.493'670'000'0 |
nm |
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_172_s |
Stable |
¹⁷²Yb |
Boson |
70 |
p |
102 |
n |
0 |
1 |
171.936'381'469'0 |
u |
21.830'000 |
% |
37.533'712'074'7 |
-59.260'280'000'0 |
MeV |
8.097'457'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_173_s |
Stable |
¹⁷³Yb |
Fermion |
70 |
p |
103 |
n |
5/2 |
-1 |
172.938'210'787'0 |
u |
16.130'000 |
% |
27.894'933'399'9 |
-57.556'282'000'0 |
MeV |
8.087'456'000'0 |
MeV |
-0.679'890'000'0 |
nm |
2.800'000'000'0 |
b |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_174_s |
Stable |
¹⁷⁴Yb |
Boson |
70 |
p |
104 |
n |
0 |
1 |
173.938'862'089'0 |
u |
31.830'000 |
% |
55.364'739'802'9 |
-56.949'598'000'0 |
MeV |
8.084'8.084'8.084'8.084 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_175_u |
Unstable |
¹⁷⁵Yb |
Fermion |
70 |
p |
105 |
n |
7/2 |
-1 |
174.941'276'450'0 |
u |
~ 0 |
% |
~ 0 |
-54.700'634'000'0 |
MeV |
8.070'954'000'0 |
MeV |
0.580'000'000'0 |
nm |
- |
|
1.15E-2 |
year |
361.601 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
470.060 |
keV |
¹⁷⁵Lu |
¹⁷⁵Yb > [ 100 % , β- , 470.06 keV ] > ¹⁷⁵Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁵Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_176_s |
Stable |
¹⁷⁶Yb |
Boson |
70 |
p |
106 |
n |
0 |
1 |
175.942'571'683'0 |
u |
12.760'000 |
% |
22.450'272'146'8 |
-53.494'133'000'0 |
MeV |
8.064'100'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
? |
% |
2β- |
1,083.380 |
keV |
¹⁷⁶Hf |
¹⁷⁶Yb > [ ? % , 2β- , 1,083.38 keV ] > ¹⁷⁶Hf |
|
|
α |
570.370 |
keV |
¹⁷²Er |
¹⁷⁶Yb > [ , α , 570.37 keV ] > ¹⁷²Er |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁷²Yb |
? |
% |
¹⁷⁶Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_177_u |
Unstable |
¹⁷⁷Yb |
Fermion |
70 |
p |
107 |
n |
9/2 |
1 |
176.945'260'822'0 |
u |
~ 0 |
% |
~ 0 |
-50.989'216'000'0 |
MeV |
8.049'989'000'0 |
MeV |
- |
|
- |
|
2.18E-4 |
year |
6.880 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
13,999.820 |
keV |
¹⁷⁷Lu |
¹⁷⁷Yb > [ 100 % , β- , 13,999.82 keV ] > ¹⁷⁷Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁷Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_178_u |
Unstable |
¹⁷⁸Yb |
Boson |
70 |
p |
108 |
n |
0 |
1 |
177.946'646'680'0 |
u |
~ 0 |
% |
~ 0 |
-49.698'297'000'0 |
MeV |
8.042'857'000'0 |
MeV |
- |
|
- |
|
1.39E-4 |
year |
4.392 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
644.700 |
keV |
¹⁷⁸Lu |
¹⁷⁸Yb > [ 100 % , β- , 644.7 keV ] > ¹⁷⁸Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁸Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_179_u |
Unstable |
¹⁷⁹Yb |
Fermion |
70 |
p |
109 |
n |
1/2 |
-1 |
178.950'170'000'0 |
u |
~ 0 |
% |
~ 0 |
-46.416'000'000'0 |
MeV |
8.025'000'000'0 |
MeV |
- |
|
- |
|
1.52E-5 |
year |
480.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
2,648.000 |
keV |
¹⁷⁹Lu |
¹⁷⁹Yb > [ 100 % , β- , 2,648.0 keV ] > ¹⁷⁹Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁷⁹Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_180_u |
Unstable |
¹⁸⁰Yb |
Boson |
70 |
p |
110 |
n |
0 |
1 |
179.952'330'000'0 |
u |
~ 0 |
% |
~ 0 |
-44.404'000'000'0 |
MeV |
8.014'000'000'0 |
MeV |
- |
|
- |
|
4.44E-6 |
year |
139.980 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
2,281.000 |
keV |
¹⁸⁰Lu |
¹⁸⁰Yb > [ 100 % , β- , 2,281.0 keV ] > ¹⁸⁰Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁸⁰Hf |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_181_u |
Unstable |
¹⁸¹Yb |
Fermion |
70 |
p |
111 |
n |
3/2 |
-1 |
180.956'150'000'0 |
u |
~ 0 |
% |
~ 0 |
-40.846'000'000'0 |
MeV |
7.994'000'000'0 |
MeV |
- |
|
- |
|
1.90E-6 |
year |
60.000 |
seconds ( x⁰ ) |
? |
% |
β- |
3,894.000 |
keV |
¹⁸¹Lu |
¹⁸¹Yb > [ ? % , β- , 3,894.0 keV ] > ¹⁸¹Lu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁸¹Ta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_070_yb_182_u |
Unstable |
¹⁸²Yb |
Boson |
70 |
p |
112 |
n |
? |
0 |
181.955'907'734'8 |
u |
~ 0 |
% |
~ 0 |
? |
MeV |
? |
MeV |
? |
nm |
? |
b |
5.07E-15 |
year |
160.000 |
nano-seconds ( x⁻⁹ ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|