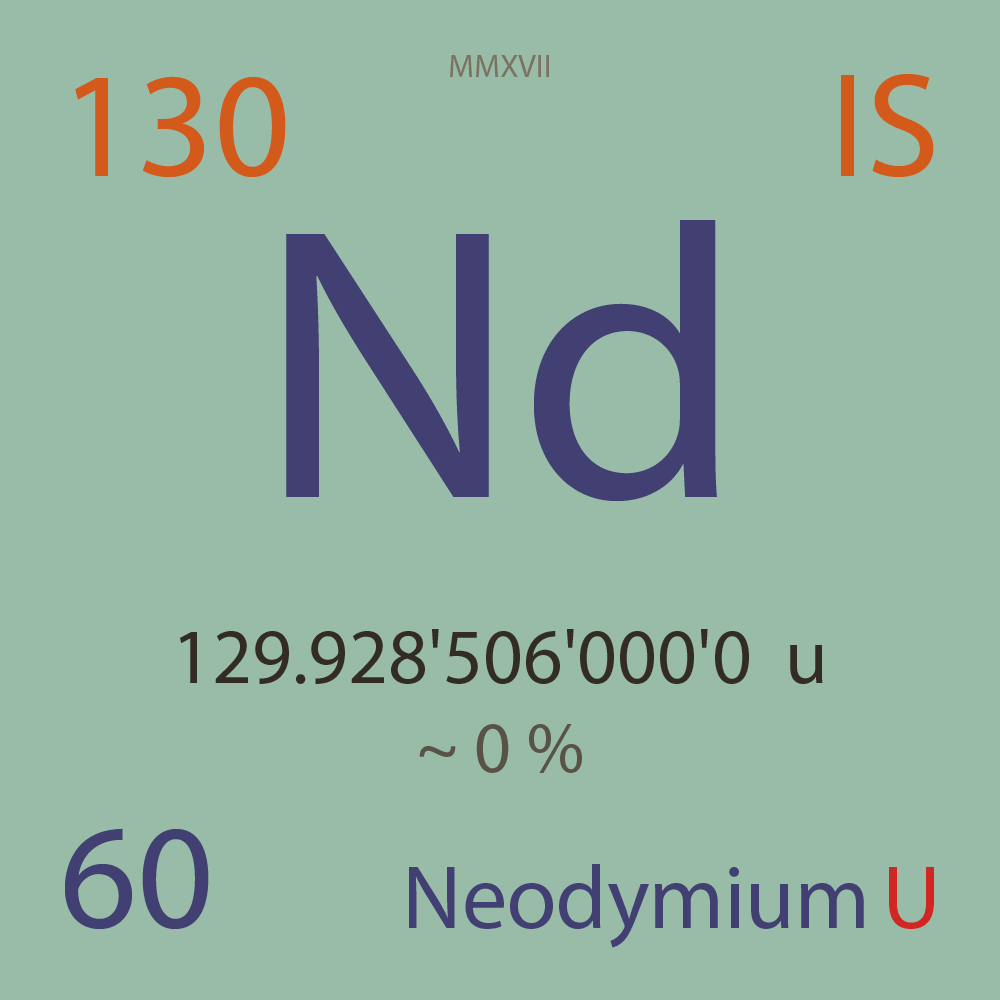| Isotope_060_nd_124_u |
Unstable |
¹²⁴Nd |
Boson |
60 |
p |
64 |
n |
0 |
1 |
123.952'230'000'0 |
u |
~ 0 |
% |
~ 0 |
-44.497'000'000'0 |
MeV |
8.052'000'000'0 |
MeV |
- |
|
- |
|
1.58E-8 |
year |
500.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β+ |
7,613.000 |
keV |
¹²⁴Pr |
¹²⁴Nd > [ ? % , β+ , 7,613.0 keV ] > ¹²⁴Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹²³Sb |
? |
% |
¹²⁴Te |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_125_u |
Unstable |
¹²⁵Nd |
Fermion |
60 |
p |
65 |
n |
5/2 |
1 |
124.948'880'000'0 |
u |
~ 0 |
% |
~ 0 |
-47.618'000'000'0 |
MeV |
8.077'000'000'0 |
MeV |
- |
|
- |
|
1.90E-8 |
year |
600.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β+ |
9,271.000 |
keV |
¹²⁵Pr |
¹²⁵Nd > [ 100 % , β+ , 9,271.0 keV ] > ¹²⁵Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹²⁵Te |
? |
% |
¹²⁴Te |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_126_u |
Unstable |
¹²⁶Nd |
Boson |
60 |
p |
66 |
n |
0 |
1 |
125.943'220'000'0 |
u |
~ 0 |
% |
~ 0 |
-52.890'000'000'0 |
MeV |
8.119'000'000'0 |
MeV |
- |
|
- |
|
3.17E-8 |
year |
1,000.000 |
milliseconds ( x⁻³ ) |
? |
% |
β+ |
6,346.000 |
keV |
¹²⁶Pr |
¹²⁶Nd > [ ? % , β+ , 6,346.0 keV ] > ¹²⁶Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹²⁵Te |
? |
% |
¹²⁶Te |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_127_u |
Unstable |
¹²⁷Nd |
Fermion |
60 |
p |
67 |
n |
5/2 |
1 |
126.940'500'000'0 |
u |
~ 0 |
% |
~ 0 |
-55.424'000'000'0 |
MeV |
8.138'000'000'0 |
MeV |
- |
|
- |
|
5.70E-8 |
year |
1.800 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
7,986.000 |
keV |
¹²⁷Pr |
¹²⁷Nd > [ 100 % , β+ , 7,986.0 keV ] > ¹²⁷Pr |
|
|
β+p |
? |
keV |
¹²⁶Ce |
¹²⁷Nd > [ , β+p , ? keV ] > ¹²⁶Ce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹²⁷I |
? |
% |
¹²⁶Te |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_128_u |
Unstable |
¹²⁸Nd |
Boson |
60 |
p |
68 |
n |
0 |
1 |
127.935'390'000'0 |
u |
~ 0 |
% |
~ 0 |
-60.184'000'000'0 |
MeV |
8.175'000'000'0 |
MeV |
- |
|
- |
|
1.58E-7 |
year |
4.980 |
seconds ( x⁰ ) |
? |
% |
β+ |
5,125.000 |
keV |
¹²⁸Pr |
¹²⁸Nd > [ ? % , β+ , 5,125.0 keV ] > ¹²⁸Pr |
|
|
β+p |
? |
keV |
¹²⁷I |
¹²⁸Nd > [ , β+p , ? keV ] > ¹²⁷I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹²⁸Xe |
? |
% |
¹²⁷I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_129_u |
Unstable |
¹²⁹Nd |
Fermion |
60 |
p |
69 |
n |
5/2 |
1 |
128.933'188'000'0 |
u |
~ 0 |
% |
~ 0 |
-62.235'000'000'0 |
MeV |
8.190'000'000'0 |
MeV |
- |
|
- |
|
1.55E-7 |
year |
4.900 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
6,517.000 |
keV |
¹²⁹Pr |
¹²⁹Nd > [ 100 % , β+ , 6,517.0 keV ] > ¹²⁹Pr |
|
|
β+p |
? |
keV |
¹²⁸Ce |
¹²⁹Nd > [ , β+p , ? keV ] > ¹²⁸Ce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹²⁹Xe |
? |
% |
¹²⁸Xe |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_130_u |
Unstable |
¹³⁰Nd |
Boson |
60 |
p |
70 |
n |
0 |
1 |
129.928'506'000'0 |
u |
~ 0 |
% |
~ 0 |
-66.596'233'000'0 |
MeV |
8.222'513'000'0 |
MeV |
- |
|
- |
|
6.65E-7 |
year |
21.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
3,557.000 |
keV |
¹³⁰Pr |
¹³⁰Nd > [ 100 % , β+ , 3,557.0 keV ] > ¹³⁰Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹³⁰Xe |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_131_u |
Unstable |
¹³¹Nd |
Fermion |
60 |
p |
71 |
n |
5/2 |
1 |
130.927'247'000'0 |
u |
~ 0 |
% |
~ 0 |
-67.768'984'000'0 |
MeV |
8.230'311'000'0 |
MeV |
- |
|
- |
|
1.05E-6 |
year |
33.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
5,487.100 |
keV |
¹³¹Pr |
¹³¹Nd > [ 100 % , β+ , 5,487.1 keV ] > ¹³¹Pr |
|
|
β+p |
? |
keV |
¹³⁰Ce |
¹³¹Nd > [ , β+p , ? keV ] > ¹³⁰Ce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹³¹Xe |
? |
% |
¹³⁰Xe |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_132_u |
Unstable |
¹³²Nd |
Boson |
60 |
p |
72 |
n |
0 |
1 |
131.923'321'237'0 |
u |
~ 0 |
% |
~ 0 |
-71.425'808'000'0 |
MeV |
8.256'810'000'0 |
MeV |
- |
|
- |
|
2.97E-6 |
year |
93.600 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
2,765.500 |
keV |
¹³²Pr |
¹³²Nd > [ 100 % , β+ , 2,765.5 keV ] > ¹³²Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹³²Xe |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_133_u |
Unstable |
¹³³Nd |
Fermion |
60 |
p |
73 |
n |
7/2 |
1 |
132.922'348'000'0 |
u |
~ 0 |
% |
~ 0 |
-72.332'373'000'0 |
MeV |
8.262'231'000'0 |
MeV |
- |
|
- |
|
2.22E-6 |
year |
70.200 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
4,583.000 |
keV |
¹³³Pr |
¹³³Nd > [ 100 % , β+ , 4,583.0 keV ] > ¹³³Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹³³Cs |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_134_u |
Unstable |
¹³⁴Nd |
Boson |
60 |
p |
74 |
n |
0 |
1 |
133.918'790'181'0 |
u |
~ 0 |
% |
~ 0 |
-75.646'459'000'0 |
MeV |
8.285'538'000'0 |
MeV |
- |
|
- |
|
1.62E-5 |
year |
510.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
1,845.500 |
keV |
¹³⁴Pr |
¹³⁴Nd > [ 100 % , β+ , 1,845.5 keV ] > ¹³⁴Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹³⁴Ba |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_135_u |
Unstable |
¹³⁵Nd |
Fermion |
60 |
p |
75 |
n |
9/2 |
-1 |
134.918'181'160'0 |
u |
~ 0 |
% |
~ 0 |
-76.213'758'000'0 |
MeV |
8.288'154'000'0 |
MeV |
0.780'000'000'0 |
nm |
2.000'000'000'0 |
b |
2.36E-5 |
year |
744.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
3,699.900 |
keV |
¹³⁵Pr |
¹³⁵Nd > [ 100 % , β+ , 3,699.9 keV ] > ¹³⁵Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹³⁵Ba |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_136_u |
Unstable |
¹³⁶Nd |
Boson |
60 |
p |
76 |
n |
0 |
1 |
135.914'976'035'0 |
u |
~ 0 |
% |
~ 0 |
-79.199'314'000'0 |
MeV |
8.308'512'000'0 |
MeV |
- |
|
- |
|
9.63E-5 |
year |
3.040 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
1,105.700 |
keV |
¹³⁶Pr |
¹³⁶Nd > [ 100 % , β+ , 1,105.7 keV ] > ¹³⁶Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹³⁶Ba |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_137_u |
Unstable |
¹³⁷Nd |
Fermion |
60 |
p |
77 |
n |
1/2 |
1 |
136.914'567'137'0 |
u |
~ 0 |
% |
~ 0 |
-79.580'199'000'0 |
MeV |
8.309'561'000'0 |
MeV |
-0.633'000'000'0 |
nm |
- |
|
7.32E-5 |
year |
2.310 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
2,574.900 |
keV |
¹³⁷Pr |
¹³⁷Nd > [ 100 % , β+ , 2,574.9 keV ] > ¹³⁷Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹³⁷Ba |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_138_u |
Unstable |
¹³⁸Nd |
Boson |
60 |
p |
78 |
n |
0 |
1 |
137.911'949'961'0 |
u |
~ 0 |
% |
~ 0 |
-82.018'083'000'0 |
MeV |
8.325'500'000'0 |
MeV |
- |
|
- |
|
5.74E-4 |
year |
18.101 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
91.200 |
keV |
¹³⁸Pr |
¹³⁸Nd > [ 100 % , β+ , 91.2 keV ] > ¹³⁸Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹³⁸Ba |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_139_u |
Unstable |
¹³⁹Nd |
Fermion |
60 |
p |
79 |
n |
3/2 |
1 |
138.911'978'288'0 |
u |
~ 0 |
% |
~ 0 |
-81.991'697'000'0 |
MeV |
8.323'482'000'0 |
MeV |
0.907'000'000'0 |
nm |
0.300'000'000'0 |
b |
5.64E-5 |
year |
1.780 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
1,809.000 |
keV |
¹³⁹Pr |
¹³⁹Nd > [ 100 % , β+ , 1,809.0 keV ] > ¹³⁹Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹³⁹La |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_140_u |
Unstable |
¹⁴⁰Nd |
Boson |
60 |
p |
80 |
n |
0 |
1 |
139.909'552'000'0 |
u |
~ 0 |
% |
~ 0 |
-84.251'770'000'0 |
MeV |
8.337'824'000'0 |
MeV |
- |
|
- |
|
9.22E-3 |
year |
290.995 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
ϵ |
443.500 |
keV |
¹⁴⁰Pr |
¹⁴⁰Nd > [ 100 % , ϵ , 443.5 keV ] > ¹⁴⁰Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁴⁰Ce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_141_u |
Unstable |
¹⁴¹Nd |
Fermion |
60 |
p |
81 |
n |
3/2 |
1 |
140.909'609'854'0 |
u |
~ 0 |
% |
~ 0 |
-84.197'879'000'0 |
MeV |
8.335'552'000'0 |
MeV |
1.013'000'000'0 |
nm |
0.340'000'000'0 |
b |
2.84E-4 |
year |
8.960 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
800.810 |
keV |
¹⁴¹Pr |
¹⁴¹Nd > [ 100 % , β+ , 800.81 keV ] > ¹⁴¹Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁴¹Pr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_142_s |
Stable |
¹⁴²Nd |
Boson |
60 |
p |
82 |
n |
0 |
1 |
141.907'723'297'0 |
u |
27.200'000 |
% |
38.598'900'736'8 |
-85.955'195'000'0 |
MeV |
8.346'066'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_143_s |
Stable |
¹⁴³Nd |
Fermion |
60 |
p |
83 |
n |
7/2 |
-1 |
142.909'814'290'0 |
u |
12.200'000 |
% |
17.434'997'343'4 |
-84.007'448'000'0 |
MeV |
8.330'524'000'0 |
MeV |
-1.065'000'000'0 |
nm |
-0.630'000'000'0 |
b |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_144_u |
Unstable |
¹⁴⁴Nd |
Boson |
60 |
p |
84 |
n |
0 |
1 |
143.910'087'274'0 |
u |
23.800'000 |
% |
34.250'600'771'2 |
-83.753'165'000'0 |
MeV |
8.326'959'000'0 |
MeV |
- |
|
- |
|
2.29E+15 |
years |
72.362 |
zetta-seconds ( x²¹ ) |
100.000'000 |
% |
α |
1,905.200 |
keV |
¹⁴⁰Ce |
¹⁴⁴Nd > [ 100 % , α , 1,905.2 keV ] > ¹⁴⁰Ce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁴⁰Ce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_145_s |
Stable |
¹⁴⁵Nd |
Fermion |
60 |
p |
85 |
n |
7/2 |
-1 |
144.912'573'636'0 |
u |
8.300'000 |
% |
12.027'743'611'8 |
-81.437'134'000'0 |
MeV |
8.309'223'000'0 |
MeV |
-0.656'000'000'0 |
nm |
-0.330'000'000'0 |
b |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_146_s |
Stable |
¹⁴⁶Nd |
Boson |
60 |
p |
86 |
n |
0 |
1 |
145.913'116'939'0 |
u |
17.200'000 |
% |
25.097'056'113'5 |
-80.931'050'000'0 |
MeV |
8.304'127'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
? |
% |
2β- |
70.830 |
keV |
¹⁴⁶Sm |
¹⁴⁶Nd > [ ? % , 2β- , 70.83 keV ] > ¹⁴⁶Sm |
|
|
α |
1,182.510 |
keV |
¹⁴²Ce |
¹⁴⁶Nd > [ , α , 1,182.51 keV ] > ¹⁴²Ce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁴²Nd |
? |
% |
¹³⁸Ba |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_147_u |
Unstable |
¹⁴⁷Nd |
Fermion |
60 |
p |
87 |
n |
5/2 |
-1 |
146.916'100'441'0 |
u |
~ 0 |
% |
~ 0 |
-78.151'936'000'0 |
MeV |
8.283'638'000'0 |
MeV |
0.578'000'000'0 |
nm |
0.900'000'000'0 |
b |
3.01E-2 |
year |
948.672 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
896.000 |
keV |
¹⁴⁷Pm |
¹⁴⁷Nd > [ 100 % , β- , 896.0 keV ] > ¹⁴⁷Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁴³Nd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_148_s |
Stable |
¹⁴⁸Nd |
Boson |
60 |
p |
88 |
n |
0 |
1 |
147.916'893'288'0 |
u |
5.700'000 |
% |
8.431'262'917'4 |
-77.413'404'000'0 |
MeV |
8.277'213'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
? |
% |
2β- |
1,928.770 |
keV |
¹⁴⁸Sm |
¹⁴⁸Nd > [ ? % , 2β- , 1,928.77 keV ] > ¹⁴⁸Sm |
|
|
α |
598.670 |
keV |
¹⁴⁴Ce |
¹⁴⁸Nd > [ , α , 598.67 keV ] > ¹⁴⁴Ce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁴⁰Ce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_149_u |
Unstable |
¹⁴⁹Nd |
Fermion |
60 |
p |
89 |
n |
5/2 |
-1 |
148.920'148'842'0 |
u |
~ 0 |
% |
~ 0 |
-74.380'875'000'0 |
MeV |
8.255'479'000'0 |
MeV |
0.351'000'000'0 |
nm |
1.300'000'000'0 |
b |
1.97E-4 |
year |
6.221 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
1,690.370 |
keV |
¹⁴⁹Pm |
¹⁴⁹Nd > [ 100 % , β- , 1,690.37 keV ] > ¹⁴⁹Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁴⁵Nd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_150_u |
Unstable |
¹⁵⁰Nd |
Boson |
60 |
p |
90 |
n |
0 |
1 |
149.920'890'888'0 |
u |
5.600'000 |
% |
8.395'569'889'7 |
-73.689'663'000'0 |
MeV |
8.249'643'000'0 |
MeV |
- |
|
- |
|
6.60E+18 |
years |
208.280 |
yotta-seconds ( x²⁴ ) |
100.000'000 |
% |
2β- |
3,367.680 |
keV |
¹⁵⁰Pm |
¹⁵⁰Nd > [ 100 % , 2β- , 3,367.68 keV ] > ¹⁵⁰Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁵⁰Sm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_151_u |
Unstable |
¹⁵¹Nd |
Fermion |
60 |
p |
91 |
n |
3/2 |
1 |
150.923'828'929'0 |
u |
~ 0 |
% |
~ 0 |
-70.952'896'000'0 |
MeV |
8.230'338'000'0 |
MeV |
- |
|
- |
|
2.37E-5 |
year |
746.400 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
2,442.340 |
keV |
¹⁵¹Pm |
¹⁵¹Nd > [ 100 % , β- , 2,442.34 keV ] > ¹⁵¹Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁵¹Eu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_152_u |
Unstable |
¹⁵²Nd |
Boson |
60 |
p |
92 |
n |
0 |
1 |
151.924'682'219'0 |
u |
~ 0 |
% |
~ 0 |
-70.158'061'000'0 |
MeV |
8.224'062'000'0 |
MeV |
- |
|
- |
|
2.18E-5 |
year |
686.400 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
1,104.200 |
keV |
¹⁵²Pm |
¹⁵²Nd > [ 100 % , β- , 1,104.2 keV ] > ¹⁵²Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁵²Sm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_153_u |
Unstable |
¹⁵³Nd |
Fermion |
60 |
p |
93 |
n |
3/2 |
-1 |
152.927'698'232'0 |
u |
~ 0 |
% |
~ 0 |
-67.348'663'000'0 |
MeV |
8.204'702'000'0 |
MeV |
- |
|
- |
|
1.00E-6 |
year |
31.600 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
3,336.000 |
keV |
¹⁵³Pm |
¹⁵³Nd > [ 100 % , β- , 3,336.0 keV ] > ¹⁵³Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁵³Eu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_154_u |
Unstable |
¹⁵⁴Nd |
Boson |
60 |
p |
94 |
n |
0 |
1 |
153.929'477'307'0 |
u |
~ 0 |
% |
~ 0 |
-65.691'466'000'0 |
MeV |
8.193'075'000'0 |
MeV |
- |
|
- |
|
8.21E-7 |
year |
25.900 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
2,807.000 |
keV |
¹⁵⁴Pm |
¹⁵⁴Nd > [ 100 % , β- , 2,807.0 keV ] > ¹⁵⁴Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁵⁴Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_155_u |
Unstable |
¹⁵⁵Nd |
Fermion |
60 |
p |
95 |
n |
3/2 |
-1 |
154.932'932'000'0 |
u |
~ 0 |
% |
~ 0 |
-62.473'000'000'0 |
MeV |
8.172'000'000'0 |
MeV |
- |
|
- |
|
2.82E-7 |
year |
8.900 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
4,500.000 |
keV |
¹⁵⁵Pm |
¹⁵⁵Nd > [ 100 % , β- , 4,500.0 keV ] > ¹⁵⁵Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁵⁵Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_156_u |
Unstable |
¹⁵⁶Nd |
Boson |
60 |
p |
96 |
n |
0 |
1 |
155.935'018'114'0 |
u |
~ 0 |
% |
~ 0 |
-60.530'237'000'0 |
MeV |
8.158'8.158'8.158'8.158 |
MeV |
- |
|
- |
|
1.74E-7 |
year |
5.490 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
3,690.000 |
keV |
¹⁵⁶Pm |
¹⁵⁶Nd > [ 100 % , β- , 3,690.0 keV ] > ¹⁵⁶Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁵⁶Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_157_u |
Unstable |
¹⁵⁷Nd |
Fermion |
60 |
p |
97 |
n |
5/2 |
-1 |
156.939'030'000'0 |
u |
~ 0 |
% |
~ 0 |
-56.793'000'000'0 |
MeV |
8.134'000'000'0 |
MeV |
- |
|
- |
|
6.34E-8 |
year |
2.000 |
seconds ( x⁰ ) |
? |
% |
β- |
5,580.000 |
keV |
¹⁵⁷Pm |
¹⁵⁷Nd > [ ? % , β- , 5,580.0 keV ] > ¹⁵⁷Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁵⁷Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_158_u |
Unstable |
¹⁵⁸Nd |
Boson |
60 |
p |
98 |
n |
0 |
1 |
157.941'600'000'0 |
u |
~ 0 |
% |
~ 0 |
-54.399'000'000'0 |
MeV |
8.119'000'000'0 |
MeV |
- |
|
- |
|
2.22E-8 |
year |
700.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
4,693.000 |
keV |
¹⁵⁸Pm |
¹⁵⁸Nd > [ ? % , β- , 4,693.0 keV ] > ¹⁵⁸Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁵⁸Gd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_159_u |
Unstable |
¹⁵⁹Nd |
Fermion |
60 |
p |
99 |
n |
7/2 |
1 |
158.946'090'000'0 |
u |
~ 0 |
% |
~ 0 |
-50.217'000'000'0 |
MeV |
8.092'000'000'0 |
MeV |
- |
|
- |
|
1.58E-8 |
year |
500.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
6,632.000 |
keV |
¹⁵⁹Pm |
¹⁵⁹Nd > [ ? % , β- , 6,632.0 keV ] > ¹⁵⁹Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁵⁹Tb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_160_u |
Unstable |
¹⁶⁰Nd |
Boson |
60 |
p |
100 |
n |
0 |
1 |
159.949'090'000'0 |
u |
~ 0 |
% |
~ 0 |
-47.422'000'000'0 |
MeV |
8.074'000'000'0 |
MeV |
- |
|
- |
|
9.51E-9 |
year |
300.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
5,682.000 |
keV |
¹⁶⁰Pm |
¹⁶⁰Nd > [ ? % , β- , 5,682.0 keV ] > ¹⁶⁰Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁶⁰Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_060_nd_161_u |
Unstable |
¹⁶¹Nd |
Fermion |
60 |
p |
101 |
n |
1/2 |
-1 |
160.953'880'000'0 |
u |
~ 0 |
% |
~ 0 |
-42.961'000'000'0 |
MeV |
8.047'000'000'0 |
MeV |
- |
|
- |
|
6.34E-9 |
year |
200.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
7,471.000 |
keV |
¹⁶¹Pm |
¹⁶¹Nd > [ ? % , β- , 7,471.0 keV ] > ¹⁶¹Pm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁶¹Dy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|