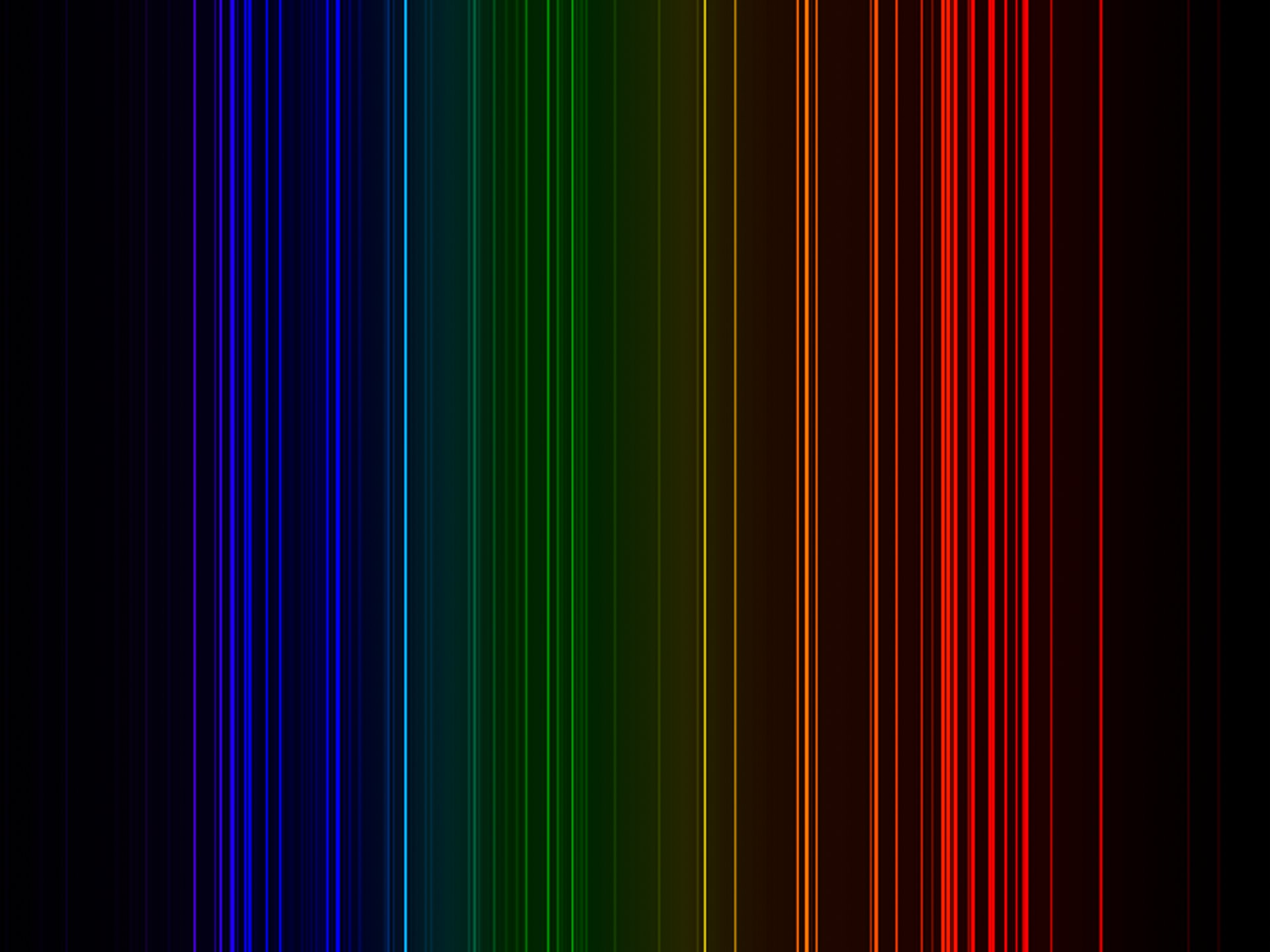
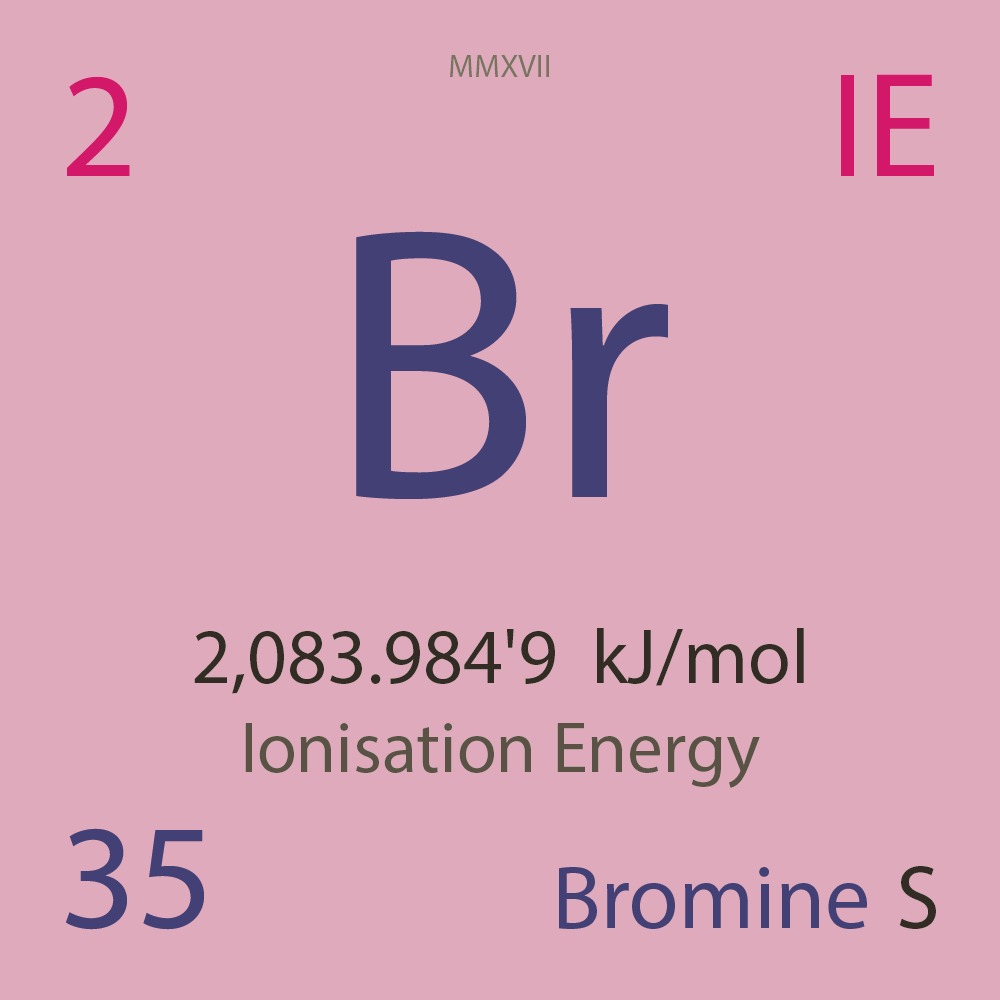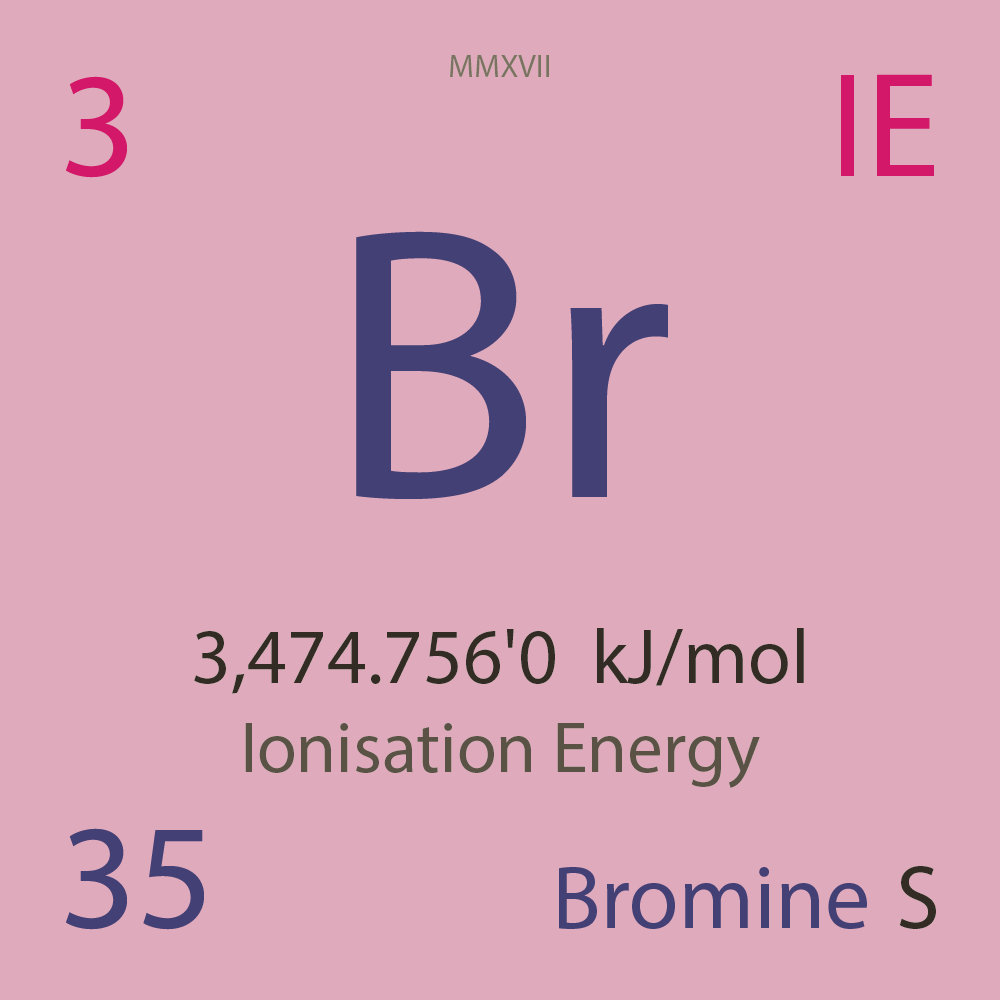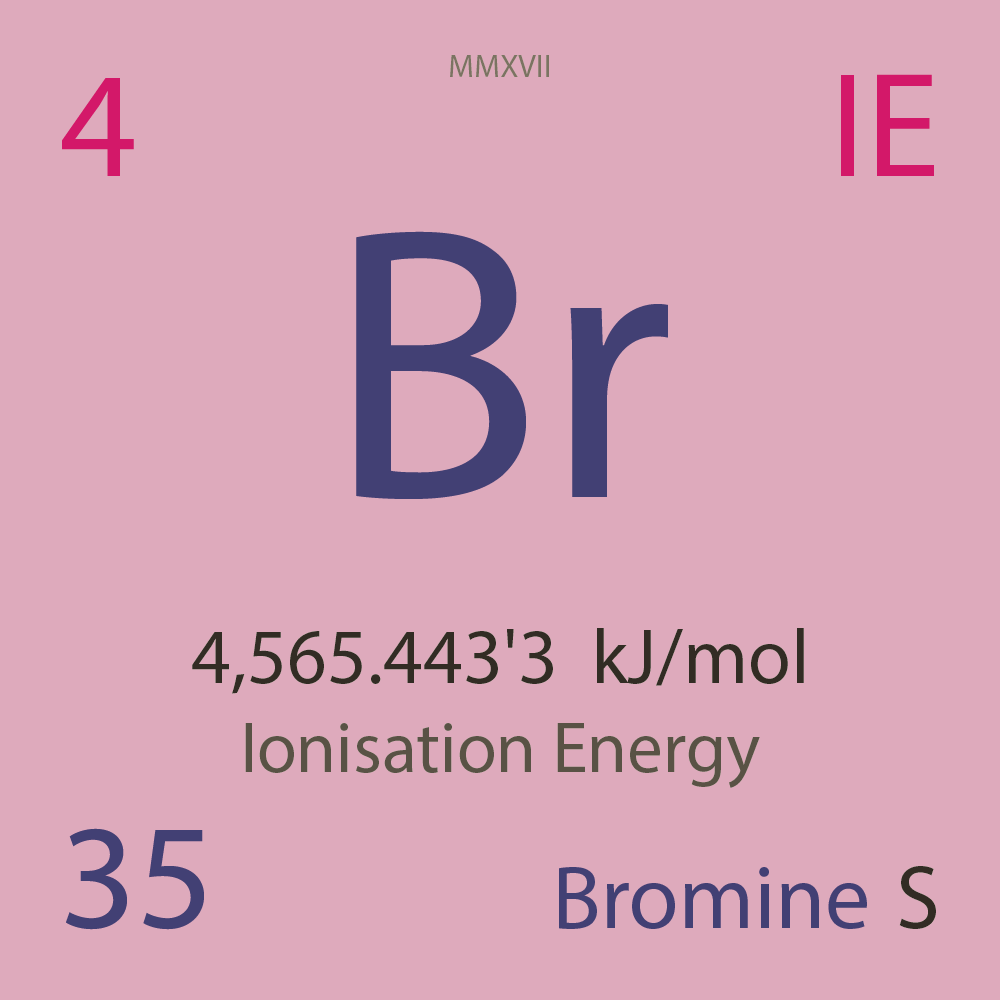| Isotope_035_br_67_u |
Unstable |
⁶⁷Br |
Boson |
35 |
p |
32 |
n |
1/2 |
-1 |
66.964'790'000'0 |
u |
~ 0 |
% |
~ 0 |
-32.798'000'000'0 |
MeV |
8.152'000'000'0 |
MeV |
- |
|
- |
|
? |
|
|
|
? |
% |
p |
1,635.000 |
keV |
⁶⁶Se |
⁶⁷Br > [ ? % , p , 1,635.0 keV ] > ⁶⁶Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁶⁶Zn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_68_u |
Unstable |
⁶⁸Br |
Fermion |
35 |
p |
33 |
n |
3 |
1 |
67.958'516'000'0 |
u |
~ 0 |
% |
~ 0 |
-38.642'000'000'0 |
MeV |
8.237'000'000'0 |
MeV |
- |
|
- |
|
4.75E-14 |
year |
1.500 |
micro-seconds ( x⁻⁶ ) |
? |
% |
p |
560.000 |
keV |
⁶⁷Se |
⁶⁸Br > [ ? % , p , 560.0 keV ] > ⁶⁷Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁶⁷Zn |
? |
% |
⁶⁶Zn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_69_u |
Unstable |
⁶⁹Br |
Boson |
35 |
p |
34 |
n |
1/2 |
-1 |
68.950'106'000'0 |
u |
~ 0 |
% |
~ 0 |
-46.476'000'000'0 |
MeV |
8.348'000'000'0 |
MeV |
- |
|
- |
|
7.61E-16 |
year |
24.000 |
nano-seconds ( x⁻⁹ ) |
? |
% |
p |
450.000 |
keV |
⁶⁸Se |
⁶⁹Br > [ ? % , p , 450.0 keV ] > ⁶⁸Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁶⁸Zn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_70_u |
Unstable |
⁷⁰Br |
Fermion |
35 |
p |
35 |
n |
0 |
1 |
69.944'792'000'0 |
u |
~ 0 |
% |
~ 0 |
-51.426'000'000'0 |
MeV |
8.415'000'000'0 |
MeV |
- |
|
- |
|
2.51E-9 |
year |
79.100 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β+ |
9,598.000 |
keV |
⁷⁰Se |
⁷⁰Br > [ 100 % , β+ , 9,598.0 keV ] > ⁷⁰Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁷⁰Ge |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_71_u |
Unstable |
⁷¹Br |
Boson |
35 |
p |
36 |
n |
5/2 |
-1 |
70.938'740'000'0 |
u |
~ 0 |
% |
~ 0 |
-57.063'323'000'0 |
MeV |
8.489'362'000'0 |
MeV |
- |
|
- |
|
6.78E-7 |
year |
21.400 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
5,031.000 |
keV |
⁷¹Se |
⁷¹Br > [ 100 % , β+ , 5,031.0 keV ] > ⁷¹Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁷¹Ga |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_72_u |
Unstable |
⁷²Br |
Fermion |
35 |
p |
37 |
n |
1 |
1 |
71.936'644'572'0 |
u |
~ 0 |
% |
~ 0 |
-59.015'201'000'0 |
MeV |
8.510'665'000'0 |
MeV |
- |
|
- |
|
2.49E-6 |
year |
78.600 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
7,857.000 |
keV |
⁷²Se |
⁷²Br > [ 100 % , β+ , 7,857.0 keV ] > ⁷²Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁷²Ge |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_73_u |
Unstable |
⁷³Br |
Boson |
35 |
p |
38 |
n |
1/2 |
-1 |
72.931'691'524'0 |
u |
~ 0 |
% |
~ 0 |
-63.628'936'000'0 |
MeV |
8.567'849'000'0 |
MeV |
- |
|
- |
|
6.27E-6 |
year |
198.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
3,566.500 |
keV |
⁷³Se |
⁷³Br > [ 100 % , β+ , 3,566.5 keV ] > ⁷³Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁷³Ge |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_74_u |
Unstable |
⁷⁴Br |
Fermion |
35 |
p |
39 |
n |
0 |
-1 |
73.929'891'034'0 |
u |
~ 0 |
% |
~ 0 |
-65.306'081'000'0 |
MeV |
8.583'803'000'0 |
MeV |
- |
|
- |
|
4.82E-5 |
year |
1.520 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
5,884.500 |
keV |
⁷⁴Se |
⁷⁴Br > [ 100 % , β+ , 5,884.5 keV ] > ⁷⁴Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁷⁴Ge |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_75_u |
Unstable |
⁷⁵Br |
Boson |
35 |
p |
40 |
n |
3/2 |
-1 |
74.925'776'207'0 |
u |
~ 0 |
% |
~ 0 |
-69.139'018'000'0 |
MeV |
8.628'8.628'8.628'8.628 |
MeV |
0.750'000'000'0 |
nm |
- |
|
1.84E-4 |
year |
5.796 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
2,007.800 |
keV |
⁷⁵Se |
⁷⁵Br > [ 100 % , β+ , 2,007.8 keV ] > ⁷⁵Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁷⁵As |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_76_u |
Unstable |
⁷⁶Br |
Fermion |
35 |
p |
41 |
n |
1 |
-1 |
75.924'541'469'0 |
u |
~ 0 |
% |
~ 0 |
-70.289'169'000'0 |
MeV |
8.635'883'000'0 |
MeV |
-0.548'210'000'0 |
nm |
0.270'000'000'0 |
b |
1.85E-3 |
year |
58.284 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
3,940.680 |
keV |
⁷⁶Se |
⁷⁶Br > [ 100 % , β+ , 3,940.68 keV ] > ⁷⁶Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁷⁶Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_77_u |
Unstable |
⁷⁷Br |
Boson |
35 |
p |
42 |
n |
3/2 |
-1 |
76.921'379'082'0 |
u |
~ 0 |
% |
~ 0 |
-73.234'914'000'0 |
MeV |
8.666'808'000'0 |
MeV |
0.920'000'000'0 |
nm |
- |
|
6.51E-3 |
year |
205.330 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
342.480 |
keV |
⁷⁷Se |
⁷⁷Br > [ 100 % , β+ , 342.48 keV ] > ⁷⁷Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁷⁷Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_78_u |
Unstable |
⁷⁸Br |
Fermion |
23 |
p |
43 |
n |
1 |
1 |
77.921'145'706'0 |
u |
~ 0 |
% |
~ 0 |
-73.452'302'000'0 |
MeV |
8.661'960'000'0 |
MeV |
- |
|
- |
|
1.23E-5 |
year |
388.020 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
2,551.580 |
keV |
⁷⁸Se |
⁷⁸Br > [ 100 % , β+ , 2,551.58 keV ] > ⁷⁸Se |
|
|
β- |
727.420 |
keV |
⁷⁸Kr |
⁷⁸Br > [ , β- , 727.42 keV ] > ⁷⁸Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁷⁸Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_79_s |
Stable |
⁷⁹Br |
Boson |
35 |
p |
44 |
n |
3/2 |
-1 |
78.918'337'087'0 |
u |
50.690'000 |
% |
40.003'705'069'4 |
-76.068'514'000'0 |
MeV |
8.687'600'000'0 |
MeV |
2.106'400'000'0 |
nm |
0.331'000'000'0 |
b |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_80_u |
Unstable |
⁸⁰Br |
Fermion |
35 |
p |
45 |
n |
1 |
1 |
79.918'529'296'0 |
u |
~ 0 |
% |
~ 0 |
-75.889'472'000'0 |
MeV |
8.677'659'000'0 |
MeV |
0.514'000'000'0 |
nm |
0.196'000'000'0 |
b |
3.36E-5 |
year |
1.061 |
kilo-seconds ( x³ ) |
91.700'000 |
% |
β- |
2,003.020 |
keV |
⁸⁰Kr |
⁸⁰Br > [ 91.7 % , β- , 2,003.02 keV ] > ⁸⁰Kr |
|
|
β+ |
848.263 |
keV |
⁸⁰Se |
⁸⁰Br > [ , β+ , 848.263 keV ] > ⁸⁰Se |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
91.700'000 |
% |
⁸⁰Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_81_s |
Stable |
⁸¹Br |
Boson |
35 |
p |
46 |
n |
3/2 |
-1 |
80.916'290'563'0 |
u |
49.310'000 |
% |
39.899'822'876'6 |
-77.974'839'000'0 |
MeV |
8.696'8.696'8.696'8.696 |
MeV |
2.270'562'000'0 |
nm |
0.276'000'000'0 |
b |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_82_u |
Unstable |
⁸²Br |
Fermion |
35 |
p |
47 |
n |
5 |
-1 |
81.916'804'119'0 |
u |
~ 0 |
% |
~ 0 |
-77.496'465'000'0 |
MeV |
8.682'468'000'0 |
MeV |
1.627'000'000'0 |
nm |
0.751'000'000'0 |
b |
4.03E-3 |
year |
127.020 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
3,093.044 |
keV |
⁸²Kr |
⁸²Br > [ 100 % , β- , 3,093.044 keV ] > ⁸²Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁸²Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_83_u |
Unstable |
⁸³Br |
Boson |
35 |
p |
48 |
n |
3/2 |
-1 |
82.915'180'421'0 |
u |
~ 0 |
% |
~ 0 |
-79.008'930'000'0 |
MeV |
8.693'327'000'0 |
MeV |
- |
|
- |
|
2.73E-4 |
year |
8.604 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
972.780 |
keV |
⁸³Kr |
⁸³Br > [ 100 % , β- , 972.78 keV ] > ⁸³Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁸³Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_84_u |
Unstable |
⁸⁴Br |
Fermion |
35 |
p |
49 |
n |
2 |
-1 |
83.916'478'974'0 |
u |
~ 0 |
% |
~ 0 |
-77.799'335'000'0 |
MeV |
8.671'522'000'0 |
MeV |
- |
|
- |
|
6.05E-5 |
year |
1.910 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
4,631.000 |
keV |
⁸⁴Kr |
⁸⁴Br > [ 100 % , β- , 4,631.0 keV ] > ⁸⁴Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁸⁴Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_85_u |
Unstable |
⁸⁵Br |
Boson |
35 |
p |
50 |
n |
3/2 |
-1 |
84.915'608'403'0 |
u |
~ 0 |
% |
~ 0 |
-78.610'267'000'0 |
MeV |
8.674'001'000'0 |
MeV |
- |
|
- |
|
5.39E-6 |
year |
169.980 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
2,870.000 |
keV |
⁸⁵Kr |
⁸⁵Br > [ 100 % , β- , 2,870.0 keV ] > ⁸⁵Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁸⁵Rb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_86_u |
Unstable |
⁸⁶Br |
Fermion |
35 |
p |
51 |
n |
2 |
-1 |
85.918'797'577'0 |
u |
~ 0 |
% |
~ 0 |
-75.639'570'000'0 |
MeV |
8.632'450'000'0 |
MeV |
- |
|
- |
|
1.75E-6 |
year |
55.098 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
7,626.000 |
keV |
⁸⁶Kr |
⁸⁶Br > [ 100 % , β- , 7,626.0 keV ] > ⁸⁶Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁸⁶Sr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_87_u |
Unstable |
⁸⁷Br |
Boson |
35 |
p |
52 |
n |
3/2 |
-1 |
86.920'711'324'0 |
u |
~ 0 |
% |
~ 0 |
-73.856'926'000'0 |
MeV |
8.605'510'000'0 |
MeV |
- |
|
- |
|
1.76E-6 |
year |
55.650 |
seconds ( x⁰ ) |
97.000'000 |
% |
β- |
6,852.500 |
keV |
⁸⁷Kr |
⁸⁷Br > [ 97 % , β- , 6,852.5 keV ] > ⁸⁷Kr |
|
|
β-n |
1,337.300 |
keV |
⁸⁶Kr |
⁸⁷Br > [ , β-n , 1,337.3 keV ] > ⁸⁶Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
97.000'000 |
% |
⁸⁷Sr |
? |
% |
⁸⁶Sr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_88_u |
Unstable |
⁸⁸Br |
Fermion |
35 |
p |
53 |
n |
? |
0 |
87.924'065'926'0 |
u |
~ 0 |
% |
~ 0 |
-70.732'135'000'0 |
MeV |
8.563'931'000'0 |
MeV |
- |
|
- |
|
5.18E-7 |
year |
16.360 |
seconds ( x⁰ ) |
93.000'000 |
% |
β- |
8,960.000 |
keV |
⁸⁸Kr |
⁸⁸Br > [ 93 % , β- , 8,960.0 keV ] > ⁸⁸Kr |
|
|
β-n |
1,906.000 |
keV |
⁸⁷Kr |
⁸⁸Br > [ , β-n , 1,906.0 keV ] > ⁸⁷Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
93.000'000 |
% |
⁸⁸Sr |
6.580'000 |
% |
⁸⁷Sr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_89_u |
Unstable |
⁸⁹Br |
Boson |
35 |
p |
54 |
n |
? |
0 |
88.926'385'334'0 |
u |
~ 0 |
% |
~ 0 |
-68.571'620'000'0 |
MeV |
8.534'120'000'0 |
MeV |
- |
|
- |
|
1.39E-7 |
year |
4.400 |
seconds ( x⁰ ) |
86.000'000 |
% |
β- |
8,155.000 |
keV |
⁸⁹Kr |
⁸⁹Br > [ 86 % , β- , 8,155.0 keV ] > ⁸⁹Kr |
|
|
β-n |
3,049.200 |
keV |
⁸⁸Kr |
⁸⁹Br > [ , β-n , 3,049.2 keV ] > ⁸⁸Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
86.000'000 |
% |
⁸⁹Y |
|
|
⁸⁸Sr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_90_u |
Unstable |
⁹⁰Br |
Fermion |
35 |
p |
55 |
n |
? |
0 |
89.930'627'737'0 |
u |
~ 0 |
% |
~ 0 |
-64.619'846'000'0 |
MeV |
8.485'069'000'0 |
MeV |
- |
|
- |
|
6.05E-8 |
year |
1.910 |
seconds ( x⁰ ) |
75.000'000 |
% |
β- |
10,350.000 |
keV |
⁹⁰Kr |
⁹⁰Br > [ 75 % , β- , 10,350.0 keV ] > ⁹⁰Kr |
|
|
β-n |
4,035.450 |
keV |
⁸⁹Kr |
⁹⁰Br > [ , β-n , 4,035.45 keV ] > ⁸⁹Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
75.000'000 |
% |
⁹⁰Zr |
25.200'000 |
% |
⁸⁹Y |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_91_u |
Unstable |
⁹¹Br |
Boson |
35 |
p |
56 |
n |
3/2 |
-1 |
90.933'968'095'0 |
u |
~ 0 |
% |
~ 0 |
-61.508'323'000'0 |
MeV |
8.446'330'000'0 |
MeV |
- |
|
- |
|
1.71E-8 |
year |
541.000 |
milli-seconds ( x⁻³ ) |
80.000'000 |
% |
β- |
9,802.000 |
keV |
⁹¹Kr |
⁹¹Br > [ 80 % , β- , 9,802.0 keV ] > ⁹¹Kr |
|
|
β-n |
5,390.200 |
keV |
⁹⁰Kr |
⁹¹Br > [ , β-n , 5,390.2 keV ] > ⁹⁰Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
80.000'000 |
% |
⁹¹Zr |
20.000'000 |
% |
⁹⁰Zr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_92_u |
Unstable |
⁹²Br |
Fermion |
35 |
p |
57 |
n |
2 |
-1 |
91.939'258'714'0 |
u |
~ 0 |
% |
~ 0 |
-56.580'144'000'0 |
MeV |
8.388'687'000'0 |
MeV |
- |
|
- |
|
1.09E-8 |
year |
343.000 |
milli-seconds ( x⁻³ ) |
67.000'000 |
% |
β- |
12,204.900 |
keV |
⁹²Kr |
⁹²Br > [ 67 % , β- , 12,204.9 keV ] > ⁹²Kr |
|
|
β-n |
6,658.900 |
keV |
⁹¹Kr |
⁹²Br > [ , β-n , 6,658.9 keV ] > ⁹¹Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
67.000'000 |
% |
⁹²Zr |
33.129'413 |
% |
⁹¹Zr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_93_u |
Unstable |
⁹³Br |
Boson |
35 |
p |
58 |
n |
3/2 |
-1 |
92.943'050'000'0 |
u |
~ 0 |
% |
~ 0 |
-53.049'000'000'0 |
MeV |
8.347'000'000'0 |
MeV |
- |
|
- |
|
3.23E-9 |
year |
102.000 |
milli-seconds ( x⁻³ ) |
68.000'000 |
% |
β-n |
7,665.000 |
keV |
⁹²Kr |
⁹³Br > [ 68 % , β-n , 7,665.0 keV ] > ⁹²Kr |
|
|
β- |
10,969.000 |
keV |
⁹³Kr |
⁹³Br > [ , β- , 10,969.0 keV ] > ⁹³Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
69.059'904 |
% |
⁹²Zr |
31.046'400 |
% |
⁹³Nb |
0.029'919 |
% |
⁹¹Zr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_94_u |
Unstable |
⁹⁴Br |
Fermion |
35 |
p |
59 |
n |
? |
0 |
93.948'680'000'0 |
u |
~ 0 |
% |
~ 0 |
-47.804'000'000'0 |
MeV |
8.289'000'000'0 |
MeV |
- |
|
- |
|
2.22E-9 |
year |
70.000 |
milli-seconds ( x⁻³ ) |
70.000'000 |
% |
β-n |
8,142.000 |
keV |
⁹³Kr |
⁹⁴Br > [ 70 % , β-n , 8,142.0 keV ] > ⁹³Kr |
|
|
β- |
13,339.000 |
keV |
⁹⁴Kr |
⁹⁴Br > [ , β- , 13,339.0 keV ] > ⁹⁴Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
71.216'640 |
% |
⁹³Nb |
2.323'169 |
% |
⁹²Zr |
0.000'146 |
% |
⁹¹Zr |
? |
% |
⁹⁴Mo |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_95_u |
Unstable |
⁹⁵Br |
Boson |
35 |
p |
60 |
n |
3/2 |
-1 |
94.952'870'000'0 |
u |
~ 0 |
% |
~ 0 |
-43.901'000'000'0 |
MeV |
8.245'000'000'0 |
MeV |
- |
|
- |
|
1.58E-9 |
year |
50.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
12,137.000 |
keV |
⁹⁵Kr |
⁹⁵Br > [ ? % , β- , 12,137.0 keV ] > ⁹⁵Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁹³Nb |
? |
% |
⁹⁵Mo |
? |
% |
⁹⁴Mo |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_96_u |
Unstable |
⁹⁶Br |
Fermion |
35 |
p |
61 |
n |
? |
0 |
95.958'530'000'0 |
u |
~ 0 |
% |
~ 0 |
-38.629'000'000'0 |
MeV |
8.188'000'000'0 |
MeV |
- |
|
- |
|
6.34E-10 |
year |
20.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
14,401.100 |
keV |
⁹⁵Kr |
⁹⁶Br > [ ? % , β- , 14,401.1 keV ] > ⁹⁵Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁹⁶Mo |
? |
% |
⁹⁵Mo |
? |
% |
⁹⁴Mo |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_035_br_97_u |
Unstable |
⁹⁷Br |
Boson |
35 |
p |
62 |
n |
3/2 |
-1 |
96.962'800'000'0 |
u |
~ 0 |
% |
~ 0 |
-34.652'000'000'0 |
MeV |
8.146'000'000'0 |
MeV |
- |
|
- |
|
3.17E-10 |
year |
10.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
13,264.000 |
keV |
⁹⁷Kr |
⁹⁷Br > [ ? % , β- , 13,264.0 keV ] > ⁹⁷Kr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁹⁷Mo |
? |
% |
⁹⁶Mo |
? |
% |
⁹⁵Mo |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|