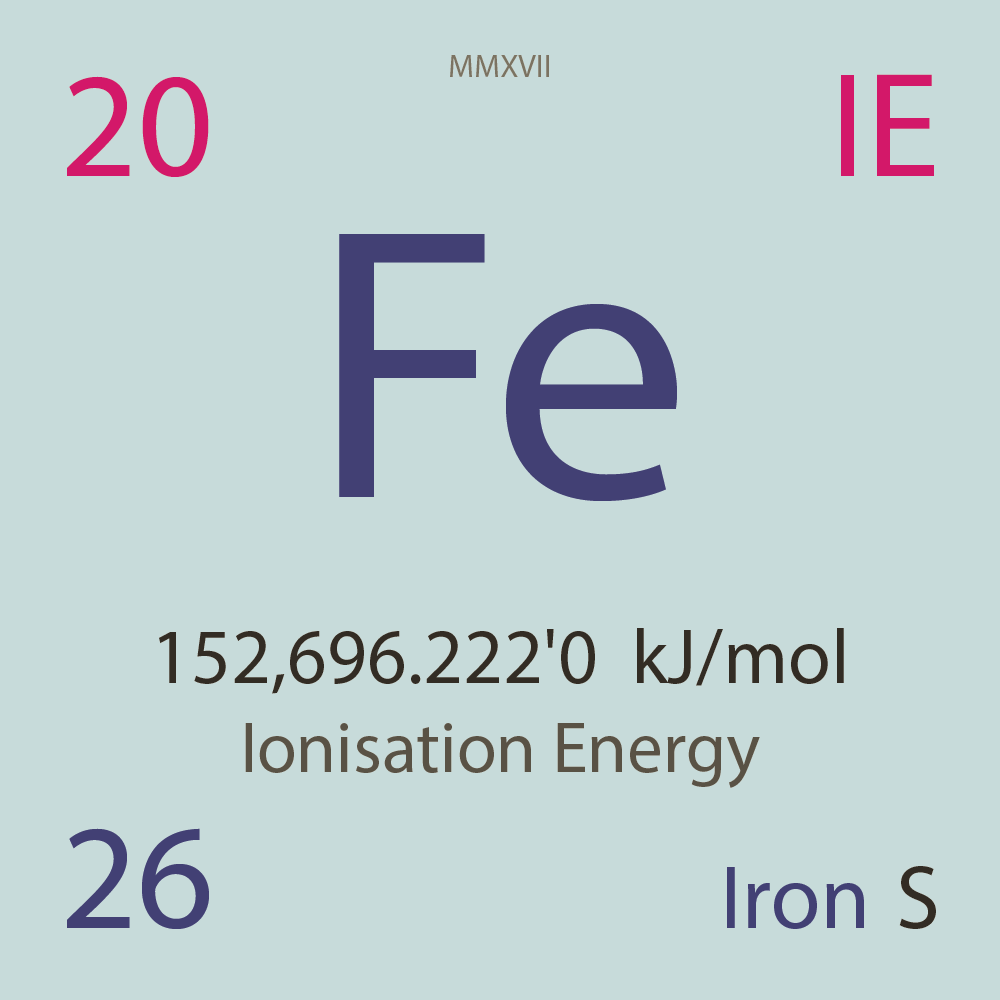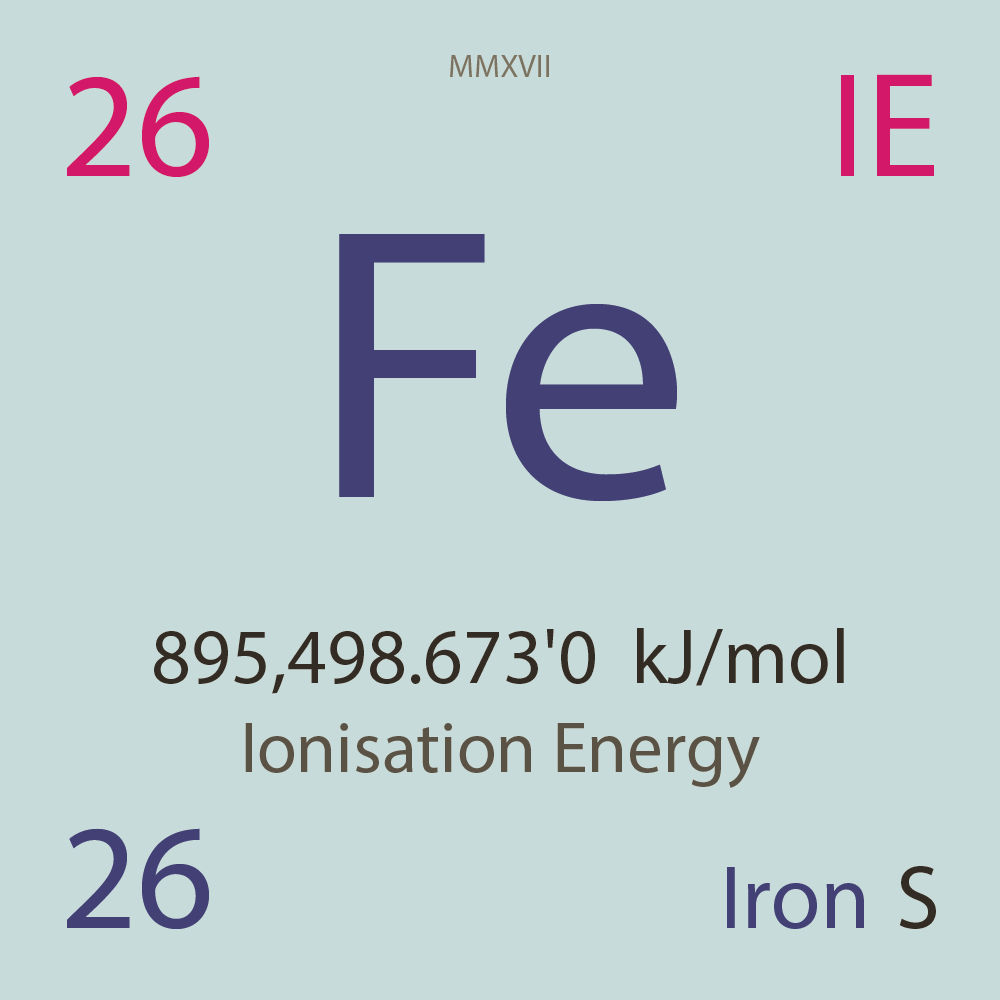| Isotope_026_fe_45_u |
Unstable |
⁴⁵Fe |
Fermion |
26 |
p |
19 |
n |
3/2 |
1 |
45.014'578'000'0 |
u |
~ 0 |
% |
~ 0 |
13.579'000'000'0 |
MeV |
7.318'000'000'0 |
MeV |
- |
|
- |
|
1.55E-10 |
year |
4.900 |
milli-seconds ( x⁻³ ) |
75.000'000 |
% |
2p |
1,134.500 |
keV |
⁴³Cr |
⁴⁵Fe > [ 75 % , 2p , 1,134.5 keV ] > ⁴³Cr |
25.000'000 |
% |
β+p |
? |
keV |
⁴⁴Cr |
⁴⁵Fe > [ 25 % , β+p , ? keV ] > ⁴⁴Cr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23.250'000 |
% |
⁴⁴Ca |
17.250'000 |
% |
⁴²Ca |
4.500'000 |
% |
⁴¹K |
1.750'000 |
% |
⁴³Ca |
? |
% |
³⁸Ar |
? |
% |
⁴⁰Ar |
? |
% |
⁴⁰Ar |
? |
% |
⁴⁰Ar |
? |
% |
⁴⁰Ar |
? |
% |
⁴⁰Ar |
? |
% |
⁴⁰Ar |
? |
% |
⁴⁰Ar |
| Isotope_026_fe_46_u |
Unstable |
⁴⁶Fe |
Boson |
26 |
p |
20 |
n |
0 |
1 |
46.000'810'000'0 |
u |
~ 0 |
% |
~ 0 |
0.755'000'000'0 |
MeV |
7.613'000'000'0 |
MeV |
- |
|
- |
|
2.85E-10 |
year |
9.000 |
milli-seconds ( x⁻³ ) |
64.000'000 |
% |
β+ |
12,103.000 |
keV |
⁴⁶Mn |
⁴⁶Fe > [ 64 % , β+ , 12,103.0 keV ] > ⁴⁶Mn |
36.000'000 |
% |
β+p |
? |
keV |
⁴⁵Cr |
⁴⁶Fe > [ 36 % , β+p , ? keV ] > ⁴⁵Cr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
40.360'000 |
% |
⁴⁵Sc |
38.400'000 |
% |
⁴⁶Ti |
21.240'000 |
% |
⁴⁴Ca |
? |
% |
⁴²Ca |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_47_u |
Unstable |
⁴⁷Fe |
Fermion |
26 |
p |
21 |
n |
7/2 |
-1 |
46.992'890'000'0 |
u |
~ 0 |
% |
~ 0 |
-6.623'000'000'0 |
MeV |
7.779'000'000'0 |
MeV |
- |
|
- |
|
6.91E-10 |
year |
21.800 |
milli-seconds ( x⁻³ ) |
87.000'000 |
% |
β+p |
? |
keV |
⁴⁶Cr |
⁴⁷Fe > [ 87 % , β+p , ? keV ] > ⁴⁶Cr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
87.000'000 |
% |
⁴⁶Ti |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_48_u |
Unstable |
⁴⁸Fe |
Boson |
26 |
p |
22 |
n |
0 |
1 |
47.980'504'000'0 |
u |
~ 0 |
% |
~ 0 |
-18.160'000'000'0 |
MeV |
8.026'000'000'0 |
MeV |
- |
|
- |
|
1.39E-9 |
year |
44.000 |
milli-seconds ( x⁻³ ) |
96.000'000 |
% |
β+ |
10,141.000 |
keV |
⁴⁸Mn |
⁴⁸Fe > [ 96 % , β+ , 10,141.0 keV ] > ⁴⁸Mn |
3.600'000 |
% |
β+p |
? |
keV |
⁴⁷Cr |
⁴⁸Fe > [ 3.6 % , β+p , ? keV ] > ⁴⁷Cr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
96.000'000 |
% |
⁴⁸Ti |
3.868'800 |
% |
⁴⁷Ti |
0.000'576 |
% |
⁴⁴Ca |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_49_u |
Unstable |
⁴⁹Fe |
Fermion |
26 |
p |
23 |
n |
7/2 |
-1 |
48.973'610'000'0 |
u |
~ 0 |
% |
~ 0 |
-24.582'000'000'0 |
MeV |
8.158'000'000'0 |
MeV |
- |
|
- |
|
2.22E-9 |
year |
70.000 |
milli-seconds ( x⁻³ ) |
52.000'000 |
% |
β+p |
? |
keV |
⁴⁸Cr |
⁴⁹Fe > [ 52 % , β+p , ? keV ] > ⁴⁸Cr |
48.000'000 |
% |
β+ |
12,011.000 |
keV |
⁴⁹Mn |
⁴⁹Fe > [ 48 % , β+ , 12,011.0 keV ] > ⁴⁹Mn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
52.000'000 |
% |
⁴⁸Ti |
48.000'000 |
% |
⁴⁹Ti |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_50_u |
Unstable |
⁵⁰Fe |
Boson |
26 |
p |
24 |
n |
0 |
1 |
49.962'988'982'0 |
u |
~ 0 |
% |
~ 0 |
-34.475'541'000'0 |
MeV |
8.354'008'000'0 |
MeV |
- |
|
- |
|
3.87E-9 |
year |
122.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β+ |
7,129.100 |
keV |
⁵⁰Mn |
⁵⁰Fe > [ 100 % , β+ , 7,129.1 keV ] > ⁵⁰Mn |
? |
% |
β+p |
? |
keV |
⁴⁹Cr |
⁵⁰Fe > [ ? % , β+p , ? keV ] > ⁴⁹Cr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁴⁹Ti |
? |
% |
⁵⁰Ti |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_51_u |
Unstable |
⁵¹Fe |
Fermion |
26 |
p |
25 |
n |
5/2 |
-1 |
50.956'819'538'0 |
u |
~ 0 |
% |
~ 0 |
-40.222'341'000'0 |
MeV |
8.461'147'000'0 |
MeV |
- |
|
- |
|
9.66E-9 |
year |
305.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β+ |
6,996.800 |
keV |
⁵¹Mn |
⁵¹Fe > [ 100 % , β+ , 6,996.8 keV ] > ⁵¹Mn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁵¹V |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_52_u |
Unstable |
⁵²Fe |
Boson |
26 |
p |
26 |
n |
0 |
1 |
51.948'113'875'0 |
u |
~ 0 |
% |
~ 0 |
-48.331'615'000'0 |
MeV |
8.609'598'000'0 |
MeV |
- |
|
- |
|
9.44E-4 |
year |
29.790 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β+ |
1,351.630 |
keV |
⁵²Mn |
⁵²Fe > [ 100 % , β+ , 1,351.63 keV ] > ⁵²Mn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁵²Cr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_53_u |
Unstable |
⁵³Fe |
Fermion |
26 |
p |
27 |
n |
7/2 |
-1 |
52.945'307'942'0 |
u |
~ 0 |
% |
~ 0 |
-50.945'323'000'0 |
MeV |
8.648'757'000'0 |
MeV |
- |
|
- |
|
1.62E-5 |
year |
511.020 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
2,720.380 |
keV |
⁵³Mn |
⁵³Fe > [ 100 % , β+ , 2,720.38 keV ] > ⁵³Mn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁵³Cr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_54_s |
Stable |
⁵⁴Fe |
Boson |
26 |
p |
28 |
n |
0 |
1 |
53.939'610'501'0 |
u |
5.845'000 |
% |
3.152'770'233'8 |
-56.252'456'000'0 |
MeV |
8.736'344'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
? |
% |
2β+ |
-1,364.310 |
keV |
⁵⁴Cr |
⁵⁴Fe > [ ? % , 2β+ , -1,364.31 keV ] > ⁵⁴Cr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁵⁴Cr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_55_u |
Unstable |
⁵⁵Fe |
Fermion |
26 |
p |
29 |
n |
3/2 |
-1 |
54.938'293'357'0 |
u |
~ 0 |
% |
~ 0 |
-57.479'368'000'0 |
MeV |
8.746'560'000'0 |
MeV |
- |
|
- |
|
2.74E+0 |
years |
86.430 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
ϵ |
231.212 |
keV |
⁵⁵Mn |
⁵⁵Fe > [ 100 % , ϵ , 231.212 keV ] > ⁵⁵Mn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁵⁵Mn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_56_s |
Stable |
⁵⁶Fe |
Boson |
26 |
p |
30 |
n |
0 |
1 |
55.934'937'475'0 |
u |
91.754'000 |
% |
51.322'542'530'8 |
-60.605'352'000'0 |
MeV |
8.790'323'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_57_s |
Stable |
⁵⁷Fe |
Fermion |
26 |
p |
31 |
n |
1/2 |
-1 |
56.935'393'969'0 |
u |
2.119'000 |
% |
1.206'460'998'2 |
-60.180'130'000'0 |
MeV |
8.770'249'000'0 |
MeV |
0.090'440'000'0 |
nm |
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_58_s |
Stable |
⁵⁸Fe |
Boson |
26 |
p |
32 |
n |
0 |
1 |
57.933'275'558'0 |
u |
0.282'000 |
% |
0.163'371'837'1 |
-62.153'418'000'0 |
MeV |
8.792'221'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_59_u |
Unstable |
⁵⁹Fe |
Fermion |
26 |
p |
33 |
n |
3/2 |
-1 |
58.934'875'464'0 |
u |
~ 0 |
% |
~ 0 |
-60.663'114'000'0 |
MeV |
8.754'743'000'0 |
MeV |
0.290'000'000'0 |
nm |
- |
|
1.22E-1 |
year |
3.844 |
mega-seconds ( x⁶ ) |
100.000'000 |
% |
β- |
1,565.297 |
keV |
⁵⁹Co |
⁵⁹Fe > [ 100 % , β- , 1,565.297 keV ] > ⁵⁹Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁵⁹Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_60_u |
Unstable |
⁶⁰Fe |
Boson |
26 |
p |
34 |
n |
0 |
1 |
59.934'071'683'0 |
u |
~ 0 |
% |
~ 0 |
-61.411'832'000'0 |
MeV |
8.755'831'000'0 |
MeV |
- |
|
- |
|
1.49E+6 |
years |
47.021 |
tera-seconds ( x¹² ) |
100.000'000 |
% |
β- |
237.180 |
keV |
⁶⁰Co |
⁶⁰Fe > [ 100 % , β- , 237.18 keV ] > ⁶⁰Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁶⁰Ni |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_61_u |
Unstable |
⁶¹Fe |
Fermion |
26 |
p |
35 |
n |
? |
0 |
60.936'745'281'0 |
u |
~ 0 |
% |
~ 0 |
-58.921'391'000'0 |
MeV |
8.703'782'000'0 |
MeV |
- |
|
- |
|
1.14E-5 |
year |
358.980 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
3,977.000 |
keV |
⁶¹Co |
⁶¹Fe > [ 100 % , β- , 3,977.0 keV ] > ⁶¹Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁶¹Ni |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_62_u |
Unstable |
⁶²Fe |
Boson |
26 |
p |
36 |
n |
0 |
1 |
61.936'767'442'0 |
u |
~ 0 |
% |
~ 0 |
-58.900'749'000'0 |
MeV |
8.693'248'000'0 |
MeV |
- |
|
- |
|
2.15E-6 |
year |
67.800 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
2,530.800 |
keV |
⁶²Co |
⁶²Fe > [ 100 % , β- , 2,530.8 keV ] > ⁶²Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁶²Ni |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_63_u |
Unstable |
⁶³Fe |
Fermion |
26 |
p |
37 |
n |
5/2 |
-1 |
62.940'369'091'0 |
u |
~ 0 |
% |
~ 0 |
-55.545'834'000'0 |
MeV |
8.630'124'000'0 |
MeV |
- |
|
- |
|
1.93E-7 |
year |
6.100 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
6,295.000 |
keV |
⁶³Co |
⁶³Fe > [ 100 % , β- , 6,295.0 keV ] > ⁶³Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁶³Cu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_64_u |
Unstable |
⁶⁴Fe |
Boson |
26 |
p |
38 |
n |
0 |
1 |
63.941'201'265'0 |
u |
~ 0 |
% |
~ 0 |
-54.770'668'000'0 |
MeV |
8.609'280'000'0 |
MeV |
- |
|
- |
|
6.34E-8 |
year |
2.000 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
5,022.000 |
keV |
⁶⁴Co |
⁶⁴Fe > [ 100 % , β- , 5,022.0 keV ] > ⁶⁴Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁶⁴Ni |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_65_u |
Unstable |
⁶⁵Fe |
Fermion |
26 |
p |
39 |
n |
1/2 |
1 |
64.945'380'270'0 |
u |
~ 0 |
% |
~ 0 |
-50.877'951'000'0 |
MeV |
8.541'116'000'0 |
MeV |
- |
|
- |
|
4.12E-8 |
year |
1.300 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
8,292.000 |
keV |
⁶⁵Co |
⁶⁵Fe > [ 100 % , β- , 8,292.0 keV ] > ⁶⁵Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁶⁵Cu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_66_u |
Unstable |
⁶⁶Fe |
Boson |
26 |
p |
40 |
n |
0 |
1 |
65.946'780'638'0 |
u |
~ 0 |
% |
~ 0 |
-49.573'517'000'0 |
MeV |
8.514'234'000'0 |
MeV |
- |
|
- |
|
1.39E-8 |
year |
440.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β- |
6,538.000 |
keV |
⁶⁶Co |
⁶⁶Fe > [ 100 % , β- , 6,538.0 keV ] > ⁶⁶Co |
? |
% |
β-n |
1,525.000 |
keV |
⁶⁵Co |
⁶⁶Fe > [ ? % , β-n , 1,525.0 keV ] > ⁶⁵Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁶⁶Zn |
? |
% |
⁶⁵Cu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_67_u |
Unstable |
⁶⁷Fe |
Fermion |
26 |
p |
41 |
n |
1/2 |
-1 |
66.950'947'244'0 |
u |
~ 0 |
% |
~ 0 |
-45.692'348'000'0 |
MeV |
8.449'695'000'0 |
MeV |
- |
|
- |
|
1.25E-8 |
year |
394.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β- |
9,369.000 |
keV |
⁶⁷Co |
⁶⁷Fe > [ 100 % , β- , 9,369.0 keV ] > ⁶⁷Co |
? |
% |
β-n |
2,348.000 |
keV |
⁶⁶Co |
⁶⁷Fe > [ ? % , β-n , 2,348.0 keV ] > ⁶⁶Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁶⁷Zn |
? |
% |
⁶⁶Zn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_68_u |
Unstable |
⁶⁸Fe |
Boson |
26 |
p |
42 |
n |
0 |
1 |
67.953'700'000'0 |
u |
~ 0 |
% |
~ 0 |
-43.128'173'000'0 |
MeV |
8.406'422'000'0 |
MeV |
- |
|
- |
|
5.93E-9 |
year |
187.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β- |
8,222.000 |
keV |
⁶⁸Co |
⁶⁸Fe > [ 100 % , β- , 8,222.0 keV ] > ⁶⁸Co |
? |
% |
β-n |
3,862.000 |
keV |
⁶⁷Co |
⁶⁸Fe > [ ? % , β-n , 3,862.0 keV ] > ⁶⁷Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
⁶⁸Zn |
? |
% |
⁶⁷Zn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_69_u |
Unstable |
⁶⁹Fe |
Fermion |
26 |
p |
43 |
n |
1/2 |
-1 |
68.958'780'000'0 |
u |
~ 0 |
% |
~ 0 |
-38.396'000'000'0 |
MeV |
8.333'000'000'0 |
MeV |
- |
|
- |
|
3.45E-9 |
year |
109.000 |
milli-seconds ( x⁻³ ) |
93.000'000 |
% |
β- |
11,606.000 |
keV |
⁶⁹Co |
⁶⁹Fe > [ 93 % , β- , 11,606.0 keV ] > ⁶⁹Co |
7.000'000 |
% |
β-n |
4,883.000 |
keV |
⁶⁸Co |
⁶⁹Fe > [ 7 % , β-n , 4,883.0 keV ] > ⁶⁸Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
92.070'000 |
% |
⁶⁹Ga |
7.930'000 |
% |
⁶⁸Zn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_70_u |
Unstable |
⁷⁰Fe |
Boson |
26 |
p |
44 |
n |
0 |
1 |
69.961'460'000'0 |
u |
~ 0 |
% |
~ 0 |
-35.900'000'000'0 |
MeV |
8.294'000'000'0 |
MeV |
- |
|
- |
|
2.98E-9 |
year |
94.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β- |
9,740.000 |
keV |
⁷⁰Co |
⁷⁰Fe > [ 100 % , β- , 9,740.0 keV ] > ⁷⁰Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁶⁹Ga |
? |
% |
⁷⁰Ge |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_71_u |
Unstable |
⁷¹Fe |
Fermion |
26 |
p |
45 |
n |
7/2 |
1 |
70.966'720'000'0 |
u |
~ 0 |
% |
~ 0 |
-31.000'000'000'0 |
MeV |
8.221'000'000'0 |
MeV |
- |
|
- |
|
9.51E-10 |
year |
30.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
12,870.000 |
keV |
⁷¹Co |
⁷¹Fe > [ ? % , β- , 12,870.0 keV ] > ⁷¹Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁷¹Ga |
? |
% |
⁷⁰Ge |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_026_fe_72_u |
Unstable |
⁷²Fe |
Boson |
26 |
p |
46 |
n |
0 |
1 |
71.969'620'000'0 |
u |
~ 0 |
% |
~ 0 |
-28.299'000'000'0 |
MeV |
8.182'000'000'0 |
MeV |
- |
|
- |
|
3.17E-10 |
year |
10.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
11,001.000 |
keV |
⁷²Co |
⁷²Fe > [ ? % , β- , 11,001.0 keV ] > ⁷²Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
⁷²Ge |
? |
% |
⁷¹Ga |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|