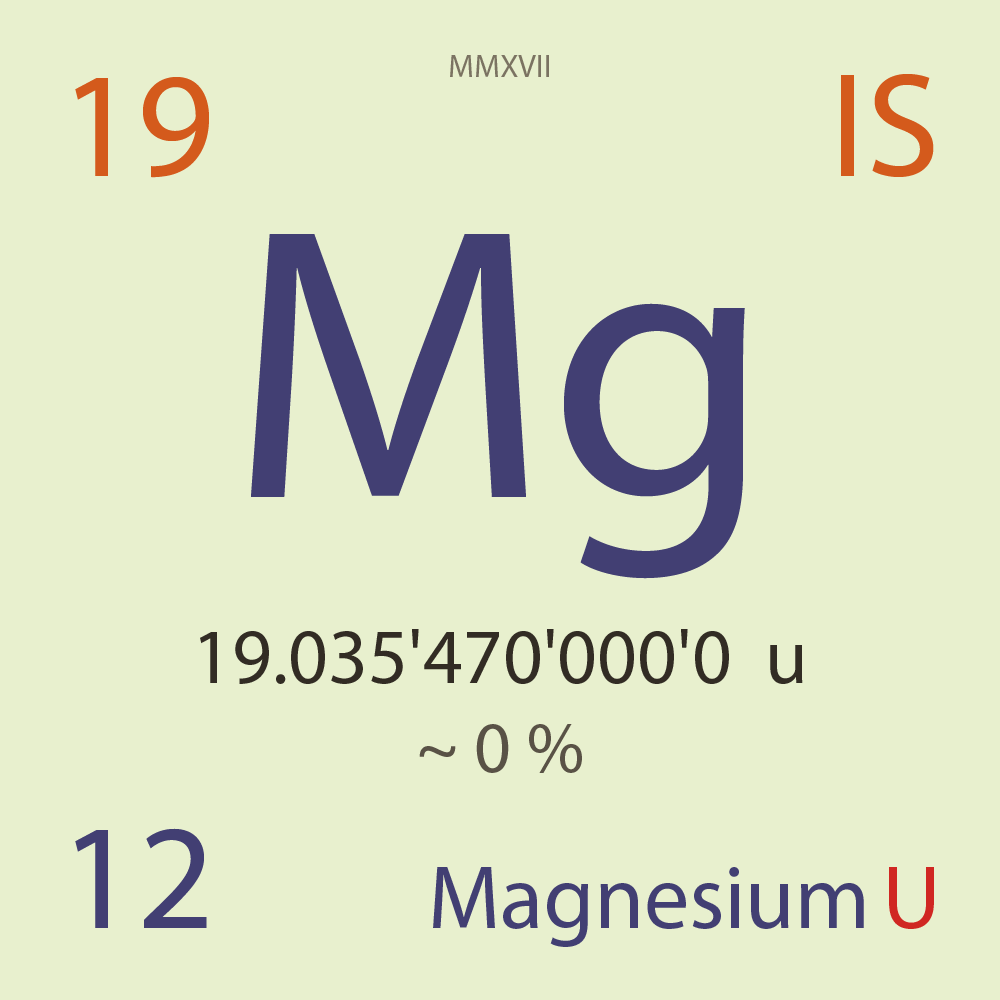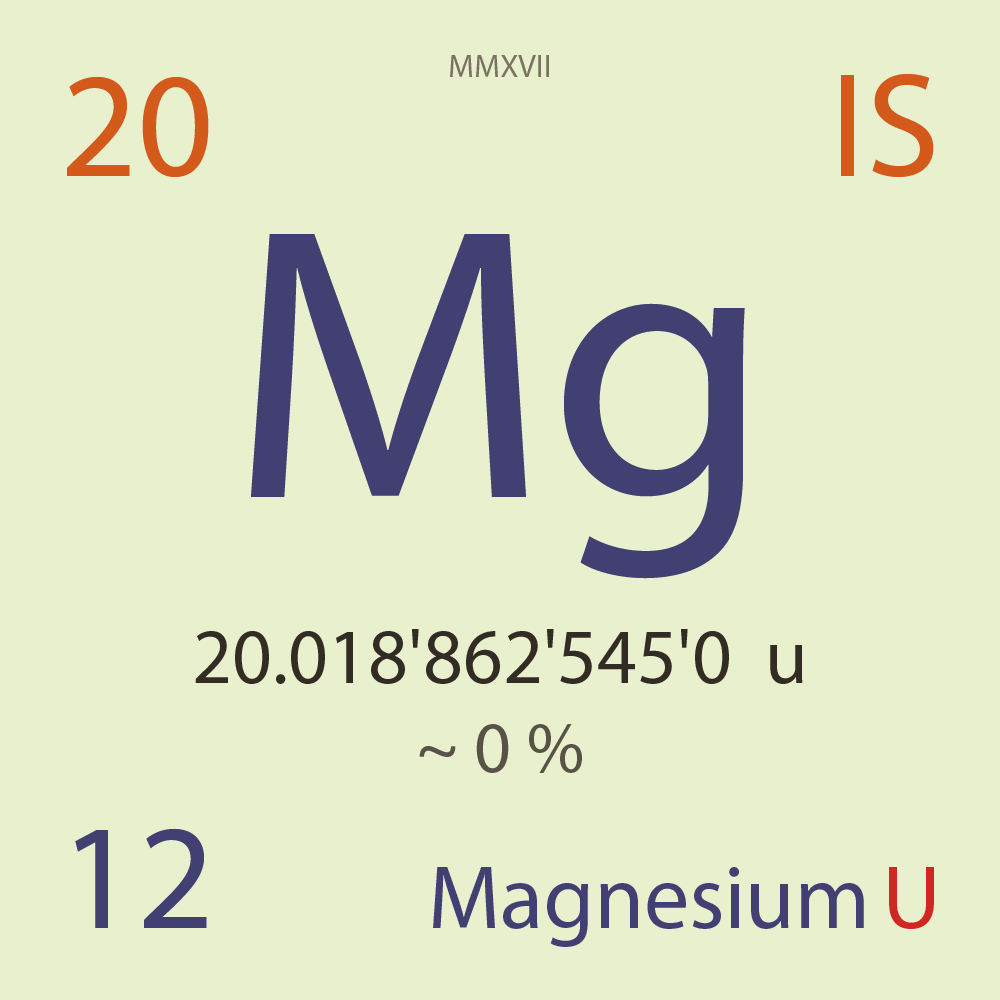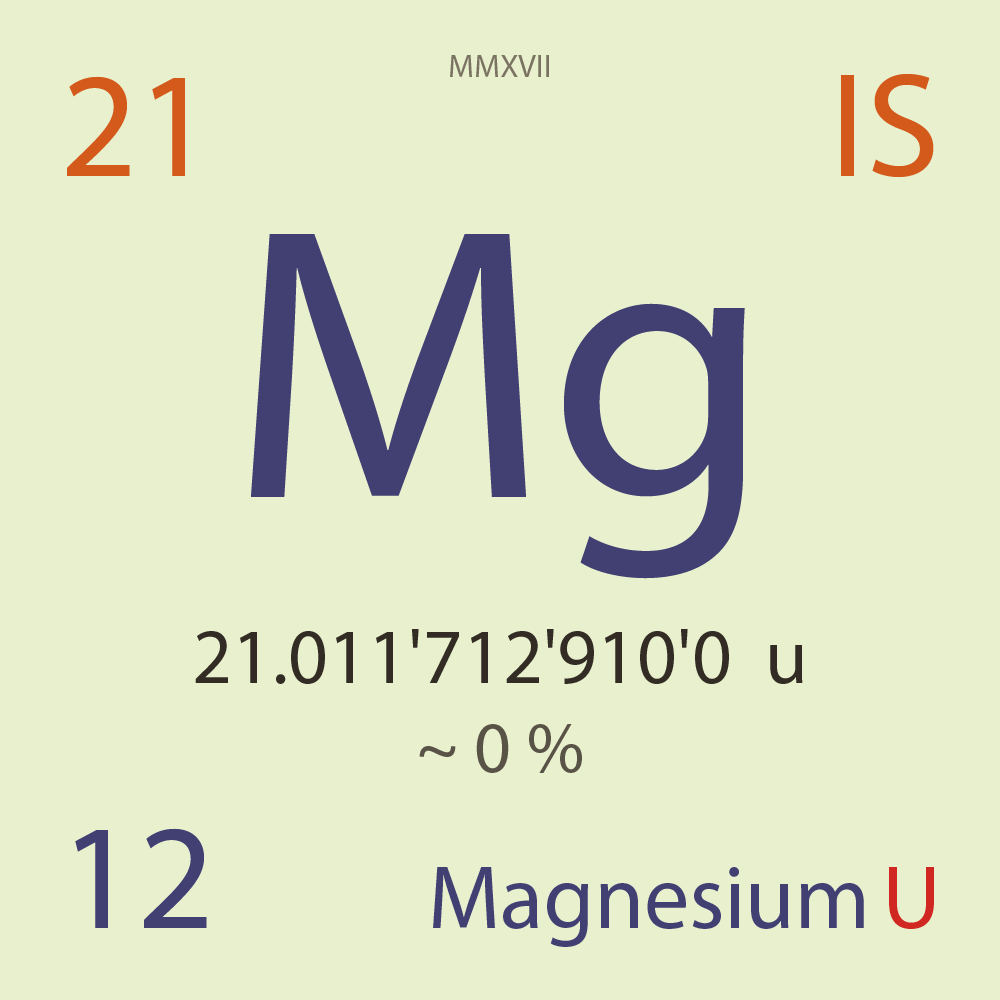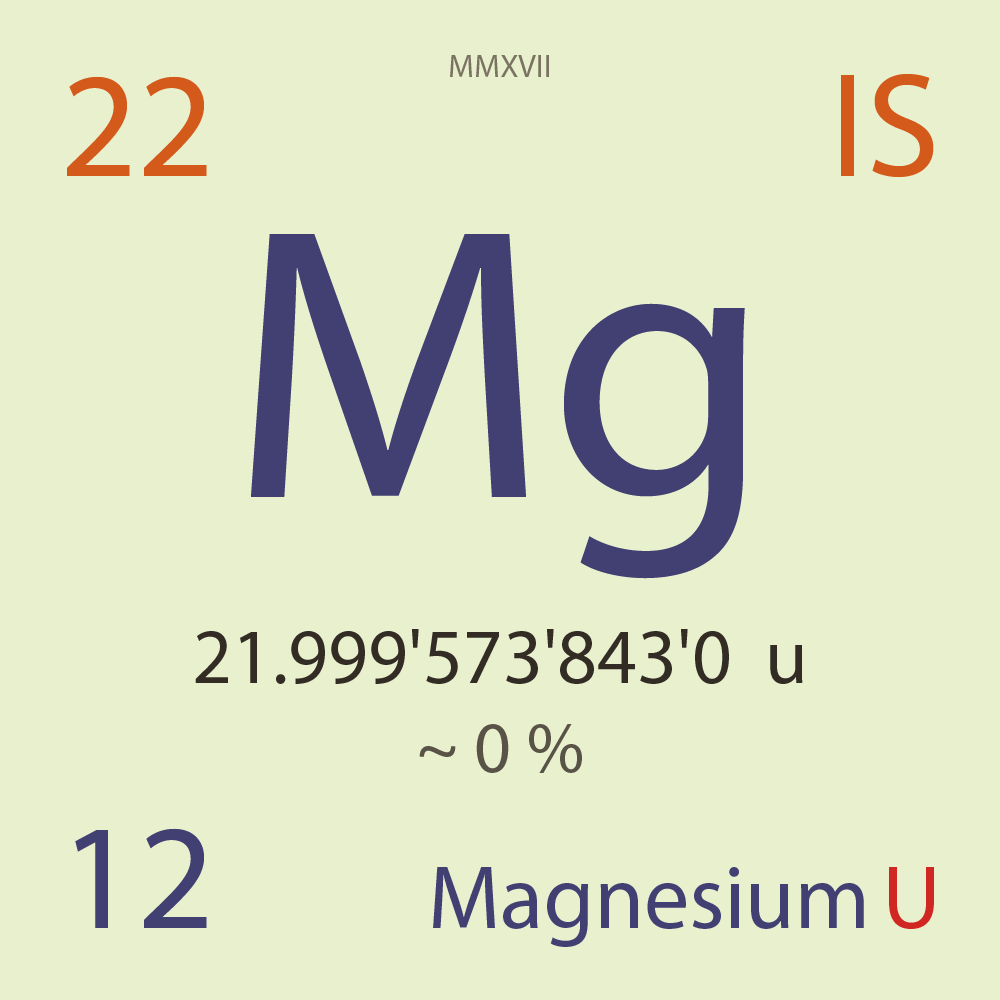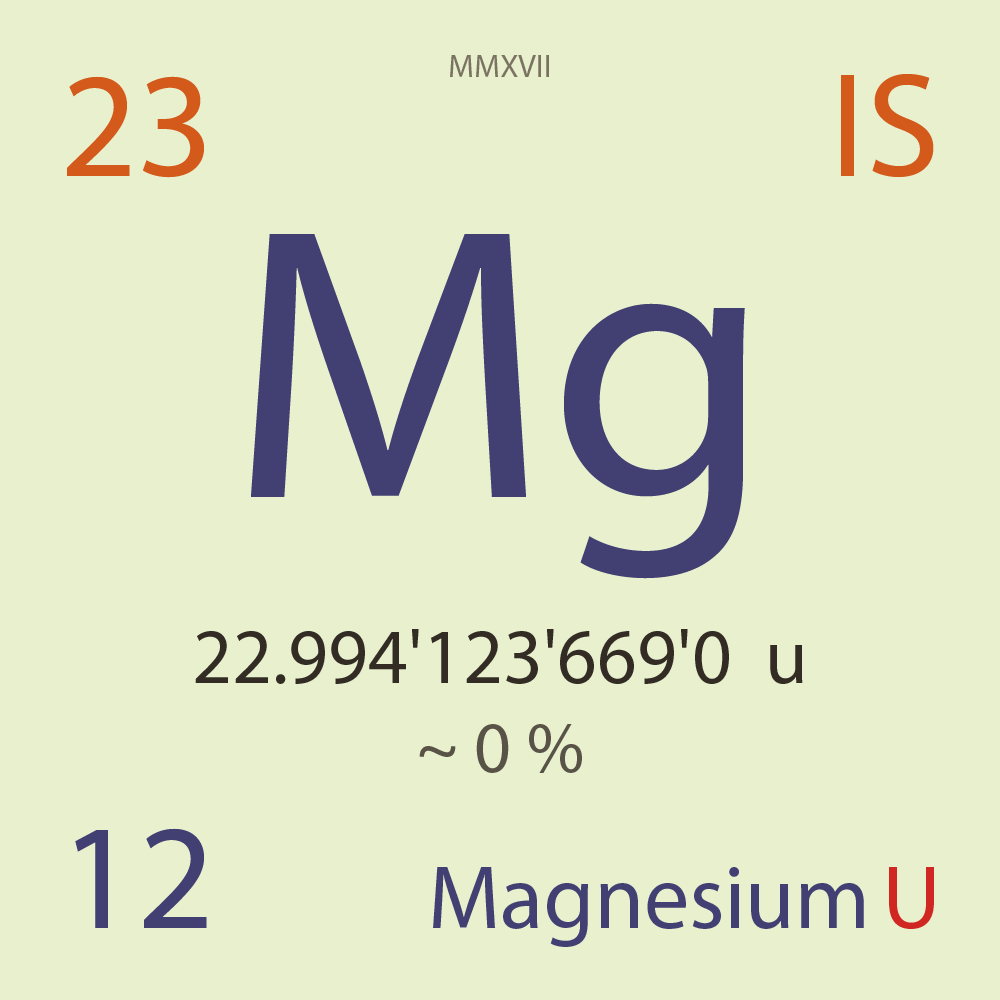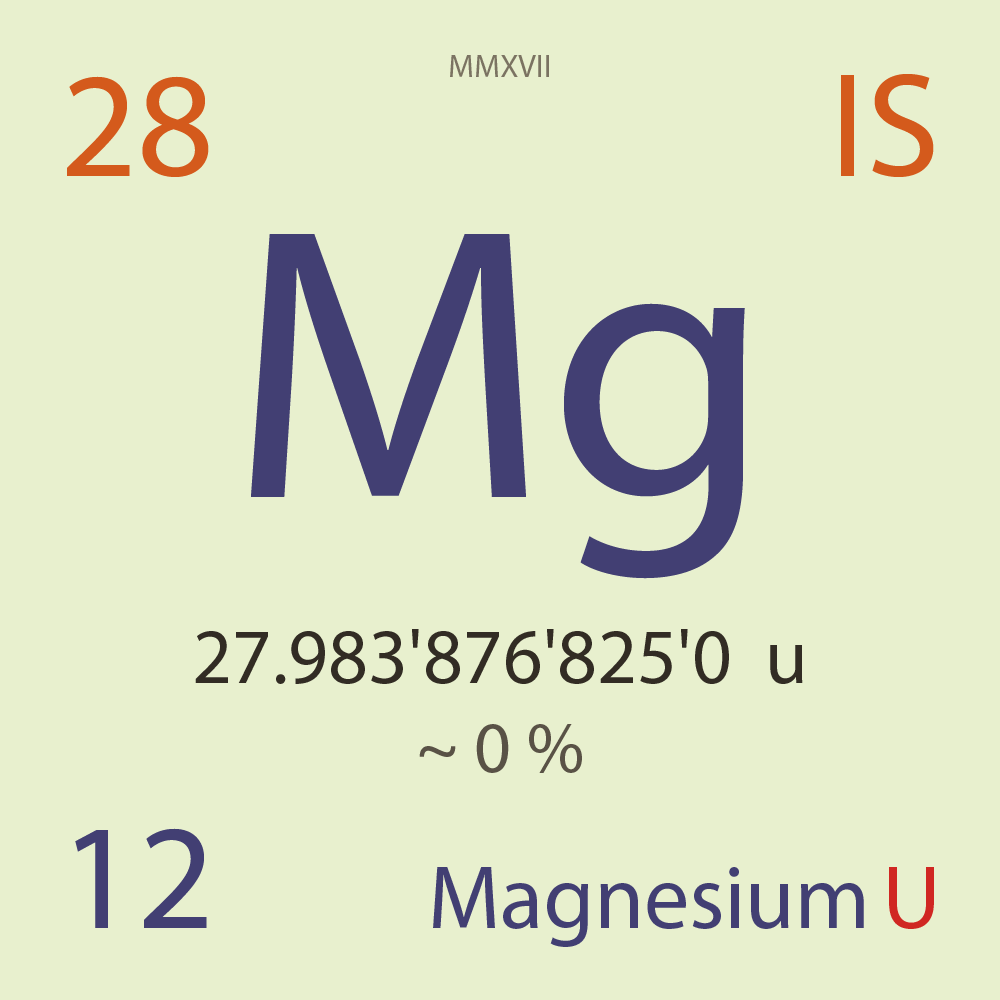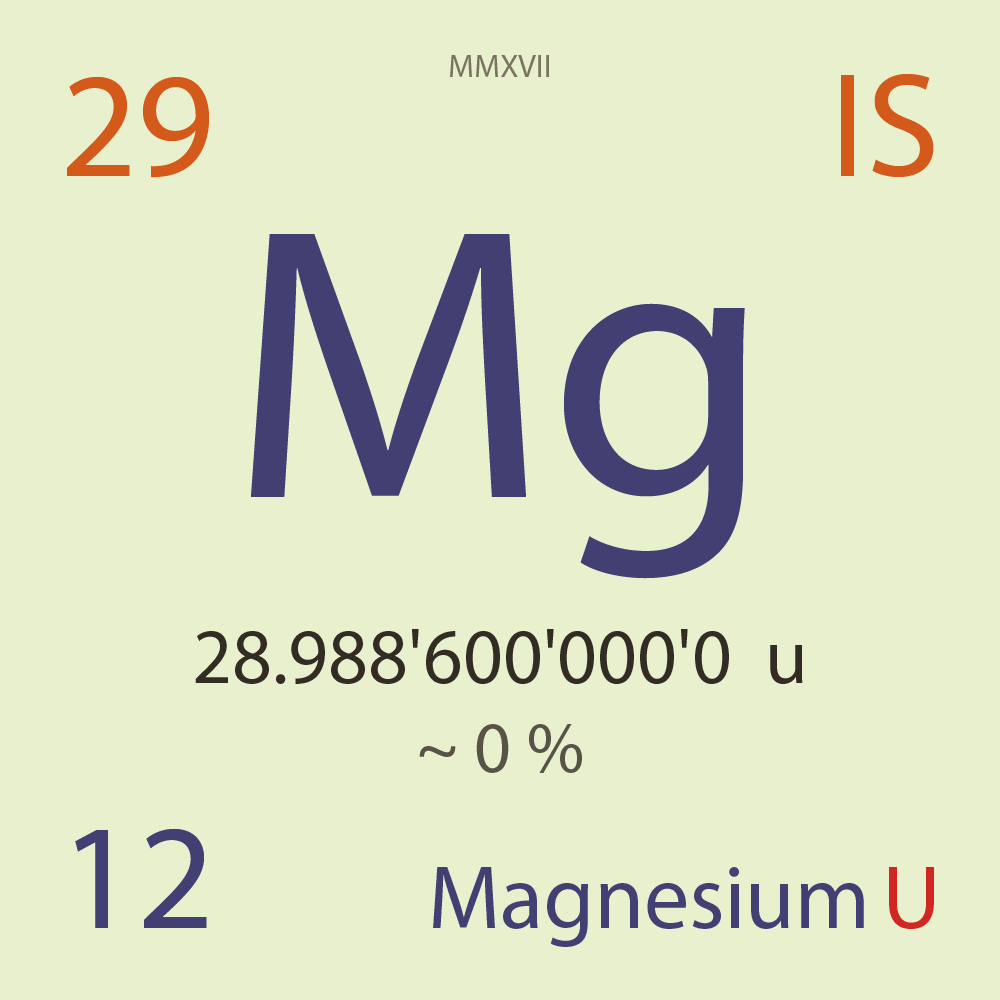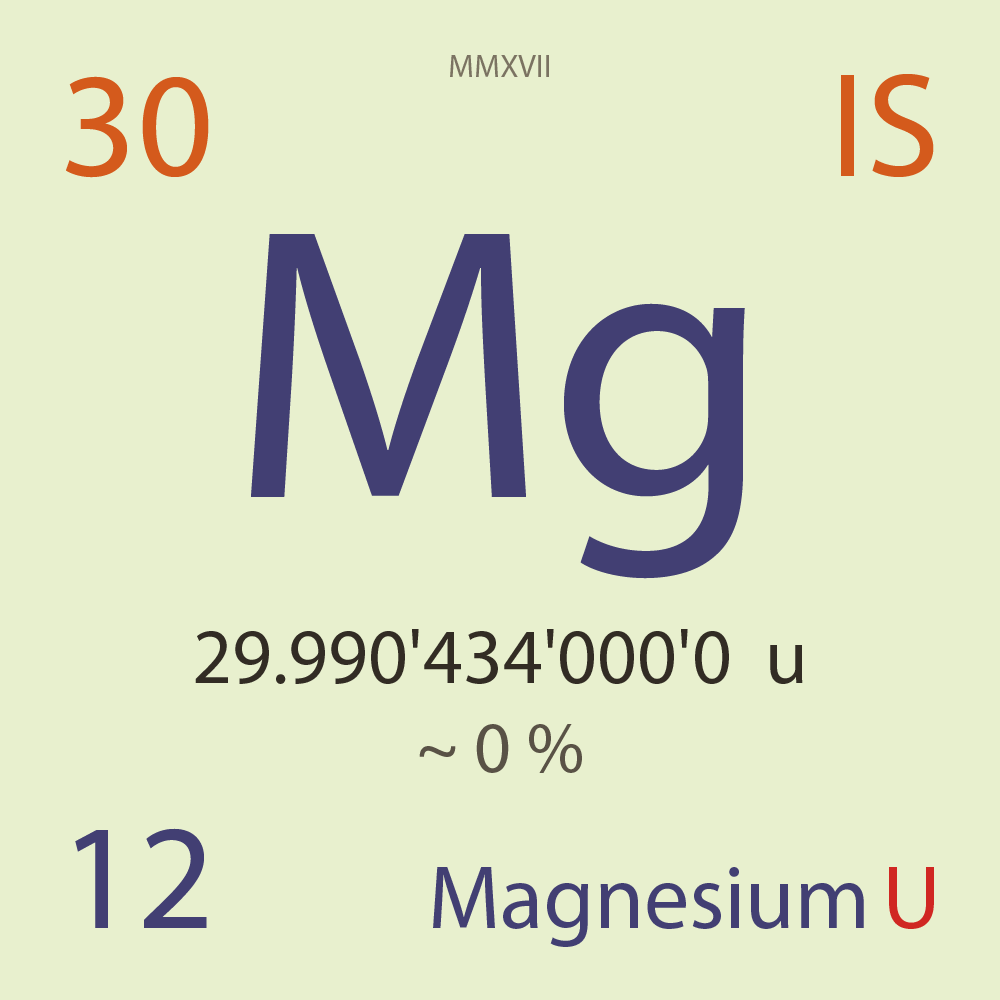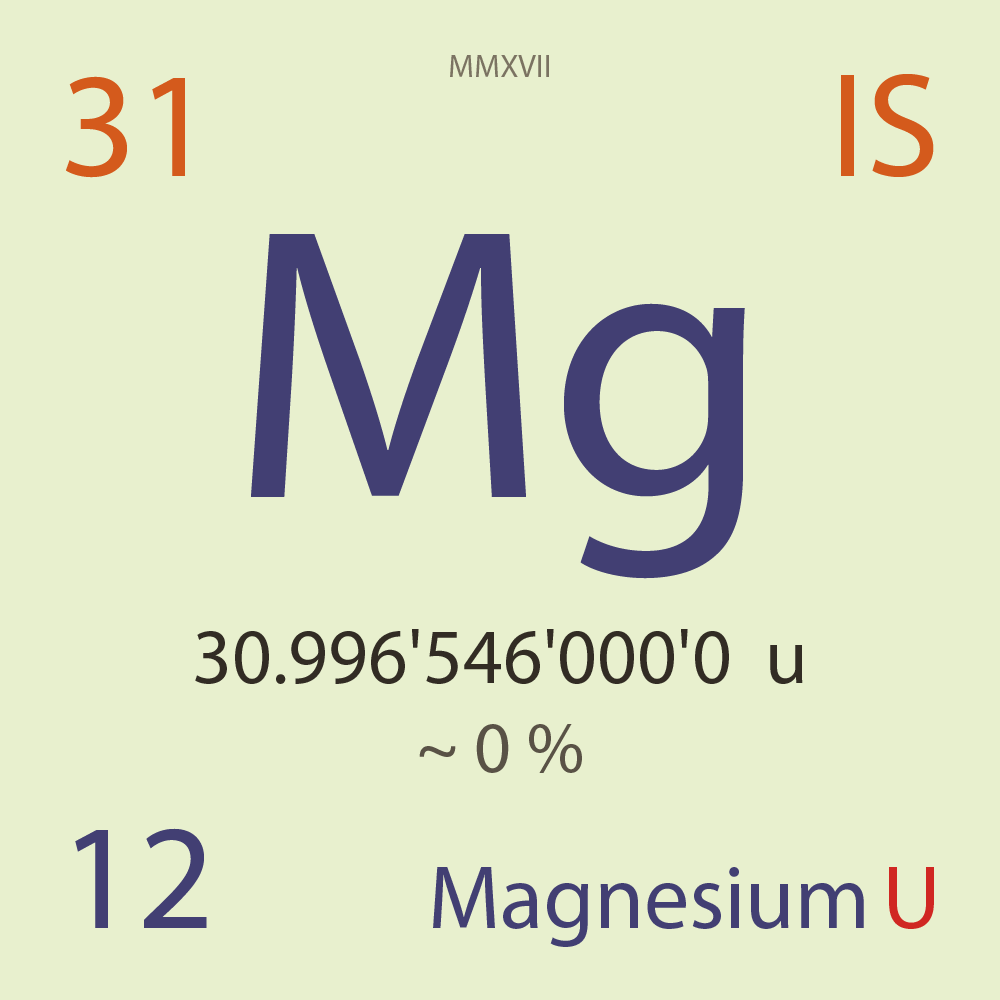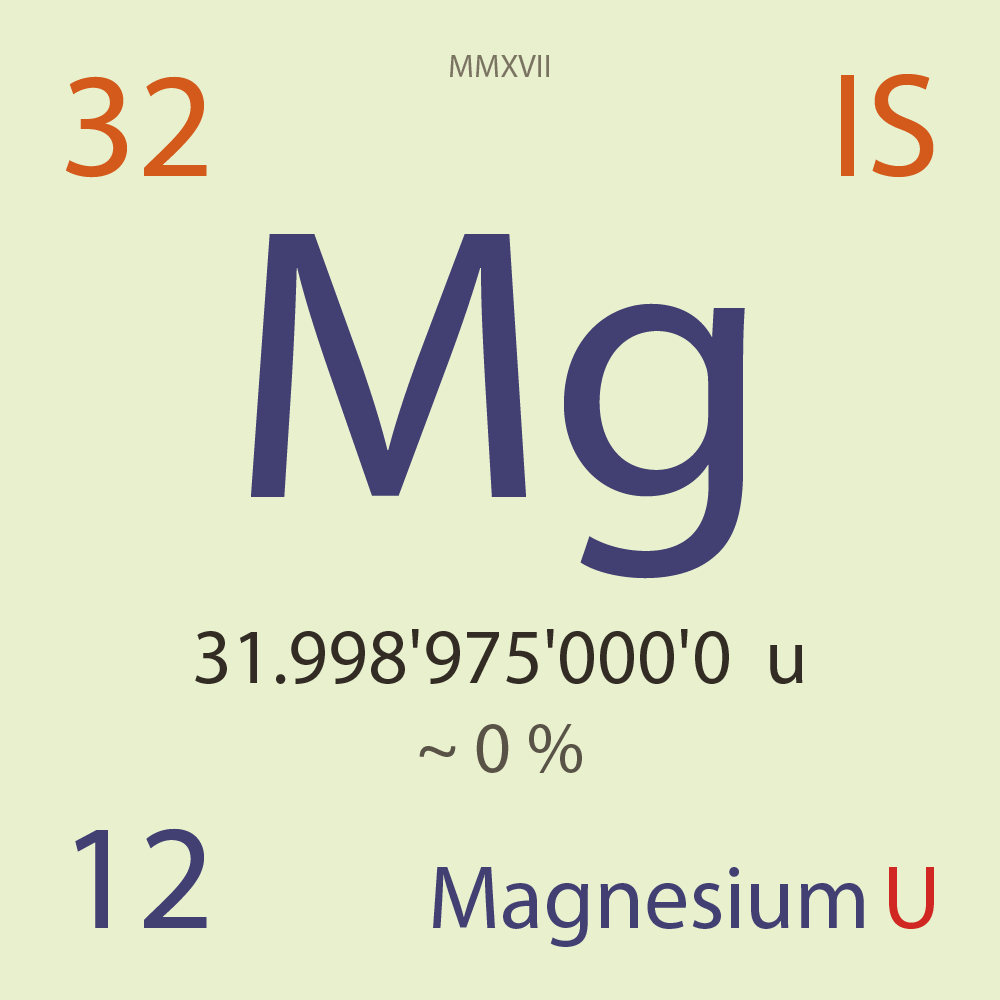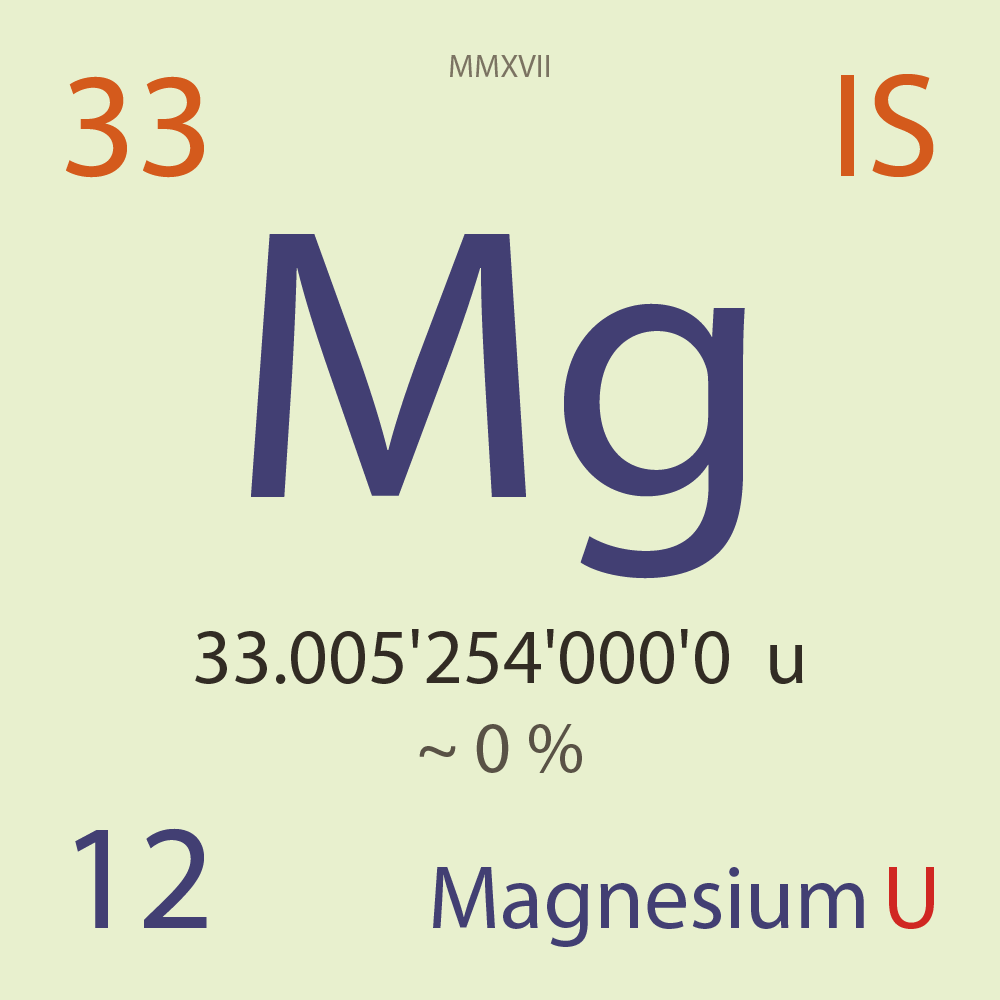| Isotope_012_mg_19_u |
Unstable |
¹⁹Mg |
Fermion |
12 |
p |
7 |
n |
1/2 |
1 |
19.035'470'000'0 |
u |
~ 0 |
% |
~ 0 |
33.040'092'000'0 |
MeV |
5.838'251'000'0 |
MeV |
- |
|
- |
|
1.27E-19 |
year |
4.000 |
pico-seconds ( x⁻¹² ) |
? |
% |
2p |
2,001.000 |
keV |
¹⁷Ne |
¹⁹Mg > [ ? % , 2p , 2,001.0 keV ] > ¹⁷Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁶O |
? |
% |
¹³C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_20_u |
Unstable |
²⁰Mg |
Boson |
12 |
p |
8 |
n |
0 |
1 |
20.018'862'545'0 |
u |
~ 0 |
% |
~ 0 |
17.570'348'000'0 |
MeV |
6.723'392'000'0 |
MeV |
- |
|
- |
|
2.85E-9 |
year |
90.000 |
milli-seconds ( x⁻³ ) |
70.000'000 |
% |
β+ |
9,700.400 |
keV |
²⁰Na |
²⁰Mg > [ 70 % , β+ , 9,700.4 keV ] > ²⁰Na |
30.400'000 |
% |
β+p |
? |
keV |
¹⁹Ne |
²⁰Mg > [ 30.4 % , β+p , ? keV ] > ¹⁹Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
52.444'490 |
% |
²⁰Ne |
30.400'000 |
% |
¹⁹F |
17.500'000 |
% |
¹⁶O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_21_u |
Unstable |
²¹Mg |
Fermion |
12 |
p |
9 |
n |
? |
1 |
21.011'712'910'0 |
u |
~ 0 |
% |
~ 0 |
10.910'506'000'0 |
MeV |
7.104'714'000'0 |
MeV |
- |
|
- |
|
3.87E-9 |
year |
122.000 |
milli-seconds ( x⁻³ ) |
67.000'000 |
% |
β+ |
12,072.000 |
keV |
²¹Na |
²¹Mg > [ 67 % , β+ , 12,072.0 keV ] > ²¹Na |
32.600'000 |
% |
β+p |
? |
keV |
²⁰Ne |
²¹Mg > [ 32.6 % , β+p , ? keV ] > ²⁰Ne |
0.005'000 |
% |
¹⁷F |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
67.000'000 |
% |
²¹Ne |
32.600'000 |
% |
²⁰Ne |
0.500'000 |
% |
¹⁶O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_22_u |
Unstable |
²²Mg |
Boson |
12 |
p |
10 |
n |
0 |
1 |
21.999'573'843'0 |
u |
~ 0 |
% |
~ 0 |
-0.396'963'000'0 |
MeV |
7.662'626'000'0 |
MeV |
- |
|
- |
|
1.22E-7 |
year |
3.857 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
3,763.270 |
keV |
²²Na |
²²Mg > [ 100 % , β+ , 3,763.27 keV ] > ²²Na |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
³⁰Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_23_u |
Unstable |
²³Mg |
Fermion |
12 |
p |
11 |
n |
3/2 |
1 |
22.994'123'669'0 |
u |
~ 0 |
% |
~ 0 |
-5.473'766'000'0 |
MeV |
7.901'126'000'0 |
MeV |
- |
|
- |
|
3.59E-7 |
year |
11.317 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
3,033.890 |
keV |
²³Na |
²³Mg > [ 100 % , β+ , 3,033.89 keV ] > ²³Na |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²³Na |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_24_s |
Stable |
²⁴Mg |
Boson |
12 |
p |
12 |
n |
0 |
1 |
23.985'041'699'0 |
u |
78.990'000 |
% |
18.945'784'438'0 |
-13.933'567'000'0 |
MeV |
8.260'709'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_25_s |
Stable |
²⁵Mg |
Fermion |
12 |
p |
13 |
n |
5/2 |
1 |
24.985'836'917'0 |
u |
10.000'000 |
% |
2.498'583'691'7 |
-13.192'826'000'0 |
MeV |
8.223'504'000'0 |
MeV |
-0.855'450'000'0 |
nm |
0.201'000'000'0 |
b |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_26_s |
Stable |
²⁶Mg |
Boson |
12 |
p |
14 |
n |
0 |
1 |
25.982'592'929'0 |
u |
11.010'000 |
% |
2.860'683'481'5 |
-16.214'582'000'0 |
MeV |
8.333'872'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_27_u |
Unstable |
²⁷Mg |
Fermion |
12 |
p |
15 |
n |
1/2 |
1 |
26.984'340'585'0 |
u |
~ 0 |
% |
~ 0 |
-14.586'651'000'0 |
MeV |
8.263'854'000'0 |
MeV |
- |
|
- |
|
1.80E-5 |
year |
567.498 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
2,610.007 |
keV |
²⁷Al |
²⁷Mg > [ 100 % , β- , 2,610.007 keV ] > ²⁷Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁷Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_28_u |
Unstable |
²⁸Mg |
Boson |
12 |
p |
16 |
n |
0 |
1 |
27.983'876'825'0 |
u |
~ 0 |
% |
~ 0 |
-15.018'641'000'0 |
MeV |
8.272'406'000'0 |
MeV |
- |
|
- |
|
2.39E-3 |
year |
75.294 |
kilo-seconds ( x³ ) |
100.000'000 |
% |
β- |
1,831.800 |
keV |
²⁸Al |
²⁸Mg > [ 100 % , β- , 1,831.8 keV ] > ²⁸Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁸Si |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_29_u |
Unstable |
²⁹Mg |
Fermion |
12 |
p |
17 |
n |
3/2 |
1 |
28.988'600'000'0 |
u |
~ 0 |
% |
~ 0 |
-10.619'032'000'0 |
MeV |
8.113'761'000'0 |
MeV |
- |
|
- |
|
4.12E-8 |
year |
1.300 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
7,596.300 |
keV |
²⁹Al |
²⁹Mg > [ 100 % , β- , 7,596.3 keV ] > ²⁹Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁹Si |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_30_u |
Unstable |
³⁰Mg |
Boson |
12 |
p |
18 |
n |
0 |
1 |
29.990'434'000'0 |
u |
~ 0 |
% |
~ 0 |
-8.910'672'000'0 |
MeV |
8.055'401'000'0 |
MeV |
- |
|
- |
|
1.06E-8 |
year |
335.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β- |
6,961.700 |
keV |
³⁰Al |
³⁰Mg > [ 100 % , β- , 6,961.7 keV ] > ³⁰Al |
0.060'000 |
% |
β-n |
1,233.330 |
keV |
³⁰Al |
³⁰Mg > [ 0.06 % , β-n , 1,233.33 keV ] > ³⁰Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
³⁰Si |
0.060'000 |
% |
²⁹Si |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_31_u |
Unstable |
³¹Mg |
Fermion |
12 |
p |
19 |
n |
3/2 |
1 |
30.996'546'000'0 |
u |
~ 0 |
% |
~ 0 |
-3.217'380'000'0 |
MeV |
7.872'260'000'0 |
MeV |
- |
|
- |
|
7.29E-9 |
year |
230.000 |
milli-seconds ( x⁻³ ) |
94.000'000 |
% |
β- |
11,736.200 |
keV |
³¹Al |
³¹Mg > [ 94 % , β- , 11,736.2 keV ] > ³¹Al |
6.200'000 |
% |
β-n |
4,583.700 |
keV |
³⁰Al |
³¹Mg > [ 6.2 % , β-n , 4,583.7 keV ] > ³⁰Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
92.119'900 |
% |
³¹P |
7.704'000 |
% |
²⁸Si |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_32_u |
Unstable |
³²Mg |
Boson |
12 |
p |
20 |
n |
0 |
1 |
31.998'975'000'0 |
u |
~ 0 |
% |
~ 0 |
-0.954'781'000'0 |
MeV |
7.807'774'000'0 |
MeV |
- |
|
- |
|
3.01E-9 |
year |
95.000 |
milli-seconds ( x⁻³ ) |
98.000'000 |
% |
β- |
10,107.200 |
keV |
³²Al |
³²Mg > [ 98 % , β- , 10,107.2 keV ] > ³²Al |
2.400'000 |
% |
β-n |
5,927.500 |
keV |
³⁰Al |
³²Mg > [ 2.4 % , β-n , 5,927.5 keV ] > ³⁰Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
97.020'000 |
% |
³²P |
3.038'000 |
% |
³¹P |
0.038'400 |
% |
³⁰Si |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_33_u |
Unstable |
³³Mg |
Fermion |
12 |
p |
21 |
n |
7/2 |
-1 |
33.005'254'000'0 |
u |
~ 0 |
% |
~ 0 |
4.894'070'000'0 |
MeV |
7.638'522'000'0 |
MeV |
- |
|
- |
|
2.87E-9 |
year |
90.500 |
milli-seconds ( x⁻³ ) |
83.000'000 |
% |
β- |
13,423.400 |
keV |
³³Al |
³³Mg > [ 83 % , β- , 13,423.4 keV ] > ³³Al |
17.000'000 |
% |
β-n |
7,884.700 |
keV |
³²Al |
³³Mg > [ 17 % , β-n , 7,884.7 keV ] > ³²Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
76.360'000 |
% |
³³S |
23.885'000 |
% |
³²S |
0.119'000 |
% |
³¹P |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_34_u |
Unstable |
³⁴Mg |
Boson |
12 |
p |
22 |
n |
0 |
1 |
34.009'456'424'0 |
u |
~ 0 |
% |
~ 0 |
8.808'603'000'0 |
MeV |
7.536'118'000'0 |
MeV |
- |
|
- |
|
6.34E-10 |
year |
20.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β- |
11,741.000 |
keV |
³⁴Al |
³⁴Mg > [ 100 % , β- , 11,741.0 keV ] > ³⁴Al |
? |
% |
β-n |
9,267.000 |
keV |
³³Al |
³⁴Mg > [ ? % , β-n , 9,267.0 keV ] > ³³Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
88.000'000 |
% |
³⁴S |
12.500'000 |
% |
³³S |
? |
% |
³²S |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_35_u |
Unstable |
³⁵Mg |
Boson |
12 |
p |
23 |
n |
7/2 |
1 |
35.017'340'000'0 |
u |
~ 0 |
% |
~ 0 |
16.152'000'000'0 |
MeV |
7.342'000'000'0 |
MeV |
- |
|
- |
|
2.22E-9 |
year |
70.000 |
milli-seconds ( x⁻³ ) |
52.000'000 |
% |
β-n |
11,013.000 |
keV |
³⁴Al |
³⁵Mg > [ 52 % , β-n , 11,013.0 keV ] > ³⁴Al |
48.000'000 |
% |
β- |
16,282.000 |
keV |
³⁵Al |
³⁵Mg > [ 48 % , β- , 16,282.0 keV ] > ³⁵Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
66.856'000 |
% |
³⁴S |
26.904'000 |
% |
³⁵Cl |
6.500'000 |
% |
³³S |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_36_u |
Unstable |
³⁶Mg |
Boson |
12 |
p |
24 |
n |
0 |
1 |
36.023'000'000'0 |
u |
~ 0 |
% |
~ 0 |
21.424'000'000'0 |
MeV |
7.215'000'000'0 |
MeV |
- |
|
- |
|
1.58E-10 |
year |
5.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
15,642.000 |
keV |
³⁶Al |
³⁶Mg > [ ? % , β- , 15,642.0 keV ] > ³⁶Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
³⁶S |
? |
% |
³⁴S |
? |
% |
³⁵Cl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_37_u |
Unstable |
³⁷Mg |
Fermion |
12 |
p |
25 |
n |
7/2 |
-1 |
37.031'400'000'0 |
u |
~ 0 |
% |
~ 0 |
29.249'000'000'0 |
MeV |
7.027'000'000'0 |
MeV |
- |
|
- |
|
1.27E-9 |
year |
40.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
19,303.000 |
keV |
³⁷Al |
³⁷Mg > [ ? % , β- , 19,303.0 keV ] > ³⁷Al |
? |
% |
β-n |
15,396.000 |
keV |
³⁷Al |
³⁷Mg > [ ? % , β-n , 15,396.0 keV ] > ³⁷Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
³⁶S |
? |
% |
³⁴S |
? |
% |
³⁷Cl |
? |
% |
³⁵Cl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_38_u |
Unstable |
³⁸Mg |
Boson |
12 |
p |
26 |
n |
0 |
1 |
38.037'570'000'0 |
u |
~ 0 |
% |
~ 0 |
34.996'000'000'0 |
MeV |
6.903'000'000'0 |
MeV |
- |
|
- |
|
3.17E-11 |
year |
1.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
18,946.000 |
keV |
³⁸Al |
³⁸Mg > [ ? % , β- , 18,946.0 keV ] > ³⁸Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
³⁷Cl |
? |
% |
³⁸Ar |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_39_u |
Unstable |
³⁹Mg |
Fermion |
12 |
p |
27 |
n |
7/2 |
-1 |
39.046'772'000'0 |
u |
~ 0 |
% |
~ 0 |
43.568'000'000'0 |
MeV |
6.713'000'000'0 |
MeV |
- |
|
- |
|
8.24E-15 |
year |
260.000 |
nano-seconds ( x⁻⁹ ) |
? |
% |
n |
500.000 |
keV |
³⁸Al |
³⁹Mg > [ ? % , n , 500.0 keV ] > ³⁸Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
³⁷Cl |
? |
% |
³⁸Ar |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_012_mg_40_u |
Unstable |
⁴⁰Mg |
Boson |
12 |
p |
28 |
n |
0 |
1 |
40.053'930'000'0 |
u |
~ 0 |
% |
~ 0 |
50.235'000'000'0 |
MeV |
6.581'000'000'0 |
MeV |
- |
|
- |
|
3.17E-11 |
year |
1.000 |
milli-seconds ( x⁻³ ) |
? |
% |
β- |
20,940.000 |
keV |
⁴⁰Al |
⁴⁰Mg > [ ? % , β- , 20,940.0 keV ] > ⁴⁰Al |
? |
% |
β-n |
20,770.000 |
keV |
³⁹Al |
⁴⁰Mg > [ ? % , β-n , 20,770.0 keV ] > ³⁹Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
³⁹K |
? |
% |
⁴⁰Ar |
? |
% |
³⁸Ar |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|