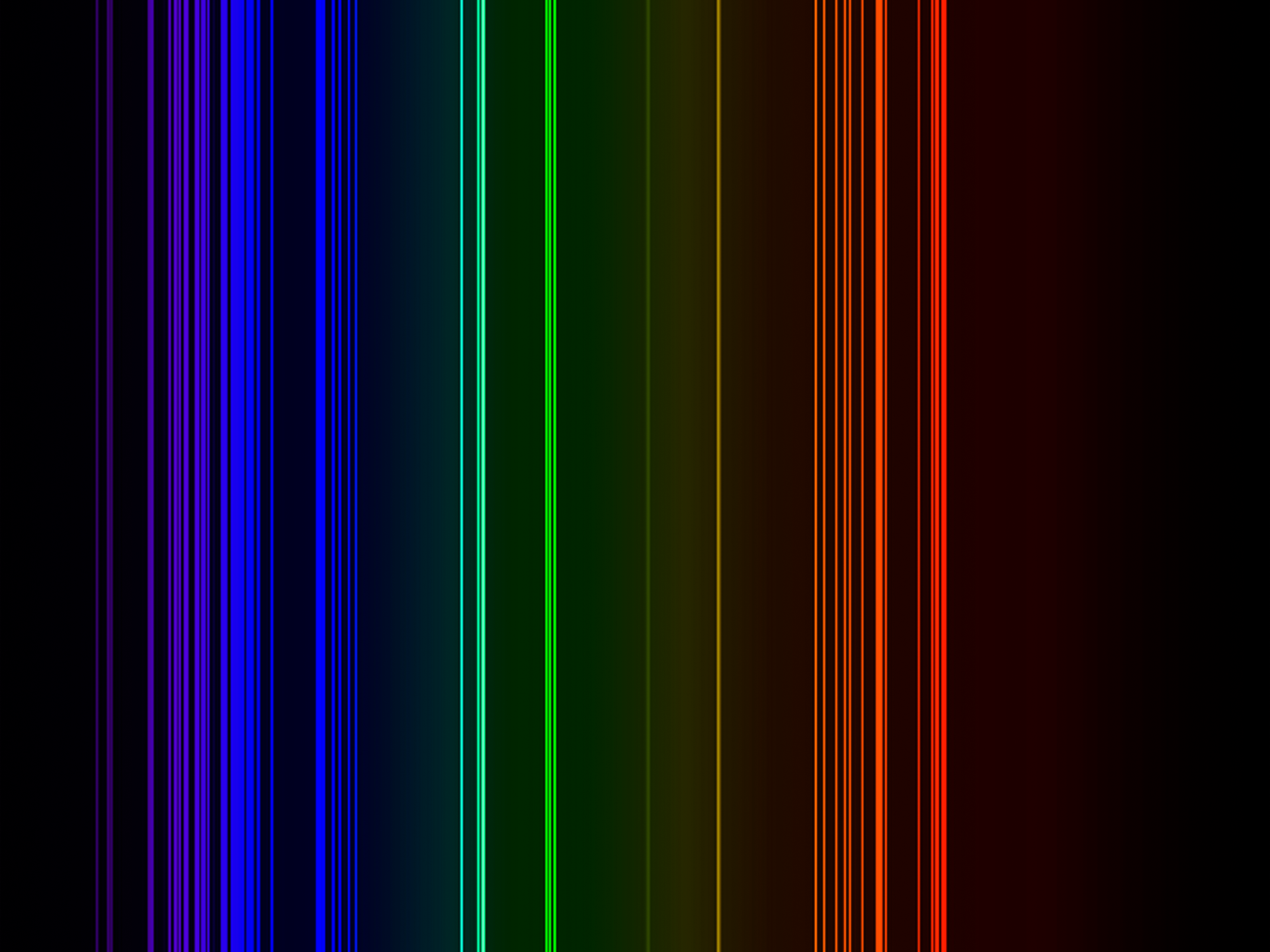
| Isotope_011_na_18_u |
Unstable |
¹⁸Na |
fermion |
11 |
p |
7 |
n |
1 |
-1 |
18.025'969'000'0 |
u |
~ 0 |
% |
~ 0 |
24.189'968'000'0 |
MeV |
6.249'329'000'0 |
MeV |
- |
|
- |
|
4.12E-29 |
year |
1.300 |
zepto-seconds ( x⁻²¹ ) |
? |
% |
p |
440.100 |
keV |
¹⁷Ne |
¹⁸Na > [ ? % , p , 440.1 keV ] > ¹⁷Ne |
? |
% |
β+ |
17,850.600 |
keV |
¹⁸Ne |
¹⁸Na > [ ? % , β+ , 17,850.6 keV ] > ¹⁸Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
¹⁸O |
? |
% |
¹⁶O |
? |
% |
¹³C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_19_u |
Unstable |
¹⁹Na |
Boson |
11 |
p |
8 |
n |
5/2 |
1 |
19.013'877'499'0 |
u |
~ 0 |
% |
~ 0 |
12.926'808'000'0 |
MeV |
6.938'021'000'0 |
MeV |
- |
|
- |
|
1.27E-15 |
year |
40.000 |
nano-seconds ( x⁻⁹ ) |
100.000'000 |
% |
p |
320.700 |
keV |
¹⁸Ne |
¹⁹Na > [ 100 % , p , 320.7 keV ] > ¹⁸Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁸O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_20_u |
Unstable |
²⁰Na |
Fermion |
11 |
p |
9 |
n |
2 |
1 |
20.007'351'328'0 |
u |
~ 0 |
% |
~ 0 |
6.847'719'000'0 |
MeV |
7.298'641'000'0 |
MeV |
0.369'400'000'0 |
nm |
- |
|
1.42E-8 |
year |
447.900 |
milli-seconds ( x⁻³ ) |
75.000'000 |
% |
β+ |
12,867.450 |
keV |
²⁰Ne |
²⁰Na > [ 75 % , β+ , 12,867.45 keV ] > ²⁰Ne |
25.000'000 |
% |
β+α |
? |
keV |
¹⁶O |
²⁰Na > [ 25 % , β+α , ? keV ] > ¹⁶O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
75.000'000 |
% |
²⁰Ne |
25.000'000 |
% |
¹⁶O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_21_u |
Unstable |
²¹Na |
Boson |
11 |
p |
10 |
n |
3/2 |
1 |
20.997'655'206'0 |
u |
~ 0 |
% |
~ 0 |
-2.184'161'000'0 |
MeV |
7.765'524'000'0 |
MeV |
2.386'300'000'0 |
nm |
0.050'000'000'0 |
b |
7.13E-7 |
year |
22.490 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
2,525.416 |
keV |
²¹Ne |
²¹Na > [ 100 % , β+ , 2,525.416 keV ] > ²¹Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²¹Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_22_u |
Unstable |
²²Na |
Fermion |
11 |
p |
11 |
n |
3 |
1 |
21.994'436'425'0 |
u |
~ 0 |
% |
~ 0 |
-5.182'436'000'0 |
MeV |
7.915'709'000'0 |
MeV |
1.746'000'000'0 |
nm |
- |
|
8.25E-8 |
year |
2.604 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
1,820.078 |
keV |
²²Ne |
²²Na > [ 100 % , β+ , 1,820.078 keV ] > ²²Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²²Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_23_s |
Stable |
²³Na |
Boson |
11 |
p |
12 |
n |
3/2 |
1 |
22.989'769'280'9 |
u |
100.000'000 |
% |
22.989'769'280'9 |
-9.529'853'580'0 |
MeV |
8.111'493'000'0 |
MeV |
2.217'520'000'0 |
nm |
0.100'600'000'0 |
b |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_24_u |
Unstable |
²⁴Na |
Fermion |
11 |
p |
13 |
n |
4 |
1 |
23.990'962'782'0 |
u |
~ 0 |
% |
~ 0 |
-8.418'114'000'0 |
MeV |
8.063'496'000'0 |
MeV |
1.690'300'000'0 |
nm |
- |
|
1.71E-3 |
year |
53.852 |
kilo-seconds ( x³ ) |
10.000'000 |
% |
β- |
5,515.453 |
keV |
²⁴Mg |
²⁴Na > [ 10 % , β- , 5,515.453 keV ] > ²⁴Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁴Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_25_u |
Unstable |
²⁵Na |
Boson |
11 |
p |
14 |
n |
5/2 |
1 |
24.989'953'968'0 |
u |
~ 0 |
% |
~ 0 |
-9.357'818'000'0 |
MeV |
8.101'397'000'0 |
MeV |
3.683'000'000'0 |
nm |
-0.100'000'000'0 |
b |
1.87E-6 |
year |
59.100 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
3,835.010 |
keV |
²⁵Mg |
²⁵Na > [ 100 % , β- , 3,835.01 keV ] > ²⁵Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁵Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_26_u |
Unstable |
²⁶Na |
Fermion |
11 |
p |
15 |
n |
3 |
1 |
25.992'633'000'0 |
u |
~ 0 |
% |
~ 0 |
-6.862'316'000'0 |
MeV |
8.004'260'000'0 |
MeV |
2.851'000'000'0 |
nm |
-0.080'000'000'0 |
b |
3.41E-8 |
year |
1.077 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
9,352.270 |
keV |
²⁶Mg |
²⁶Na > [ 100 % , β- , 9,352.27 keV ] > ²⁶Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁶Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_27_u |
Unstable |
²⁷Na |
Boson |
11 |
p |
16 |
n |
5/2 |
1 |
26.994'076'788'0 |
u |
~ 0 |
% |
~ 0 |
-5.517'436'000'0 |
MeV |
7.956'933'000'0 |
MeV |
3.895'000'000'0 |
nm |
0.060'000'000'0 |
b |
9.54E-9 |
year |
301.000 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β- |
9,069.210 |
keV |
²⁷Mg |
²⁷Na > [ 100 % , β- , 9,069.21 keV ] > ²⁷Mg |
0.130'000 |
% |
β-n |
2,625.830 |
keV |
²⁶Mg |
²⁷Na > [ 0.13 % , β-n , 2,625.83 keV ] > ²⁶Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁷Mg |
0.013'000 |
% |
²⁶Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_28_u |
Unstable |
²⁸Na |
Fermion |
11 |
p |
17 |
n |
1 |
1 |
27.998'938'000'0 |
u |
~ 0 |
% |
~ 0 |
-0.989'247'000'0 |
MeV |
7.799'297'000'0 |
MeV |
2.426'000'000'0 |
nm |
-0.020'000'000'0 |
b |
9.66E-10 |
year |
30.500 |
milli-seconds ( x⁻³ ) |
99.000'000 |
% |
β- |
14,029.000 |
keV |
²⁸Mg |
²⁸Na > [ 99 % , β- , 14,029.0 keV ] > ²⁸Mg |
0.580'000 |
% |
β-n |
5,526.100 |
keV |
²⁷Mg |
²⁸Na > [ 0.58 % , β-n , 5,526.1 keV ] > ²⁷Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
99.000'000 |
% |
²⁸Si |
0.580'000 |
% |
²⁷Al |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_29_u |
Unstable |
²⁹Na |
Boson |
11 |
p |
18 |
n |
3/2 |
1 |
29.002'861'000'0 |
u |
~ 0 |
% |
~ 0 |
2.665'004'000'0 |
MeV |
7.682'668'000'0 |
MeV |
2.449'000'000'0 |
nm |
0.030'000'000'0 |
b |
1.42E-9 |
year |
44.900 |
milli-seconds ( x⁻³ ) |
74.000'000 |
% |
β- |
13,284.000 |
keV |
²⁹Mg |
²⁹Na > [ 74 % , β- , 13,284.0 keV ] > ²⁹Mg |
25.900'000 |
% |
β-n |
9,612.300 |
keV |
²⁸Mg |
²⁹Na > [ 25.9 % , β-n , 9,612.3 keV ] > ²⁸Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
74.000'000 |
% |
²⁹Si |
25.900'000 |
% |
²⁸Si |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_30_u |
Unstable |
³⁰Na |
Fermion |
11 |
p |
19 |
n |
2 |
1 |
30.008'976'000'0 |
u |
~ 0 |
% |
~ 0 |
8.361'090'000'0 |
MeV |
7.505'754'000'0 |
MeV |
2.083'000'000'0 |
nm |
- |
|
1.53E-9 |
year |
48.400 |
milli-seconds ( x⁻³ ) |
69.000'000 |
% |
β- |
17,271.800 |
keV |
³⁰Mg |
³⁰Na > [ 69 % , β- , 17,271.8 keV ] > ³⁰Mg |
30.000'000 |
% |
β-n |
10,908.800 |
keV |
²⁹Mg |
³⁰Na > [ 30 % , β-n , 10,908.8 keV ] > ²⁹Mg |
0.011'700 |
% |
β-2n |
7,237.100 |
keV |
²⁸Mg |
³⁰Na > [ 0.0117 % , β-2n , 7,237.1 keV ] > ²⁸Mg |
0.000'055 |
% |
β-α |
5,506.600 |
keV |
²⁶Ne |
³⁰Na > [ 0.000055 % , β-α , 5,506.6 keV ] > ²⁶Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
69.000'000 |
% |
³⁰Si |
30.041'400 |
% |
²⁹Si |
1.170'000 |
% |
²⁸Si |
0.000'055 |
% |
²⁶Mg |
0.000'000 |
% |
²⁵Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_31_u |
Unstable |
³¹Na |
Boson |
11 |
p |
20 |
n |
3/2 |
1 |
31.013'585'452'0 |
u |
~ 0 |
% |
~ 0 |
12.654'768'000'0 |
MeV |
7.385'492'000'0 |
MeV |
2.305'000'000'0 |
nm |
- |
|
5.39E-10 |
year |
17.000 |
milli-seconds ( x⁻³ ) |
62.000'000 |
% |
β- |
15,872.000 |
keV |
³¹Mg |
³¹Na > [ 62 % , β- , 15,872.0 keV ] > ³¹Mg |
37.000'000 |
% |
β-n |
13,494.000 |
keV |
³⁰Mg |
³¹Na > [ 37 % , β-n , 13,494.0 keV ] > ³⁰Mg |
0.900'000 |
% |
β-2n |
7,131.000 |
keV |
²⁹Mg |
³¹Na > [ 0.9 % , β-2n , 7,131.0 keV ] > ²⁹Mg |
0.050'000 |
% |
β-3n |
? |
keV |
²⁸Mg |
³¹Na > [ 0.05 % , β-3n , ? keV ] > ²⁸Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
57.114'400 |
% |
³¹P |
41.776'480 |
% |
³⁰Si |
0.922'200 |
% |
²⁹Si |
0.050'000 |
% |
²⁸Si |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_32_u |
Unstable |
³²Na |
Fermion |
11 |
p |
21 |
n |
? |
0 |
32.020'466'560'0 |
u |
~ 0 |
% |
~ 0 |
19.064'478'000'0 |
MeV |
7.206'621'000'0 |
MeV |
- |
|
- |
|
4.09E-10 |
year |
12.900 |
milli-seconds ( x⁻³ ) |
68.000'000 |
% |
β- |
20,019.000 |
keV |
³²Mg |
³²Na > [ 68 % , β- , 20,019.0 keV ] > ³²Mg |
24.000'000 |
% |
β-n |
14,211.000 |
keV |
³¹Mg |
³²Na > [ 24 % , β-n , 14,211.0 keV ] > ³¹Mg |
0.080'000 |
% |
β-2n |
11,833.000 |
keV |
³⁰Mg |
³²Na > [ 0.08 % , β-2n , 11,833.0 keV ] > ³⁰Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
65.973'600 |
% |
³²P |
24.174'640 |
% |
³¹P |
9.875'072 |
% |
³⁰Si |
0.004'800 |
% |
²⁹Si |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_33_u |
Unstable |
³³Na |
Boson |
11 |
p |
22 |
n |
3/2 |
1 |
33.026'719'756'0 |
u |
~ 0 |
% |
~ 0 |
24.889'293'000'0 |
MeV |
7.056'314'000'0 |
MeV |
- |
|
- |
|
2.60E-10 |
year |
8.200 |
milli-seconds ( x⁻³ ) |
47.000'000 |
% |
β-n |
17,773.000 |
keV |
³²Mg |
³³Na > [ 47 % , β-n , 17,773.0 keV ] > ³²Mg |
40.000'000 |
% |
β- |
19,995.000 |
keV |
³³Mg |
³³Na > [ 40 % , β- , 19,995.0 keV ] > ³³Mg |
0.130'000 |
% |
β-2n |
11,964.000 |
keV |
³¹Mg |
³³Na > [ 0.13 % , β-2n , 11,964.0 keV ] > ³¹Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
55.153'400 |
% |
³²S |
30.544'000 |
% |
³³S |
13.451'060 |
% |
³¹P |
1.019'568 |
% |
³⁰Si |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_34_u |
Unstable |
³⁴Na |
Fermion |
11 |
p |
23 |
n |
1 |
1 |
34.035'170'000'0 |
u |
~ 0 |
% |
~ 0 |
32.761'000'000'0 |
MeV |
6.855'000'000'0 |
MeV |
- |
|
- |
|
1.74E-10 |
year |
5.500 |
milli-seconds ( x⁻³ ) |
50.000'000 |
% |
β-2n |
17,573.000 |
keV |
³²Mg |
³⁴Na > [ 50 % , β-2n , 17,573.0 keV ] > ³²Mg |
35.000'000 |
% |
β- |
23,952.000 |
keV |
³⁴Mg |
³⁴Na > [ 35 % , β- , 23,952.0 keV ] > ³⁴Mg |
0.150'000 |
% |
β-n |
19,795.000 |
keV |
³³Mg |
³⁴Na > [ 0.15 % , β-n , 19,795.0 keV ] > ³³Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
52.092'750 |
% |
³²S |
30.800'000 |
% |
³⁴S |
15.829'000 |
% |
³³S |
1.536'850 |
% |
³¹P |
0.019'200 |
% |
³⁰Si |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_35_u |
Unstable |
³⁵Na |
Boson |
11 |
p |
24 |
n |
3/2 |
1 |
35.042'493'000'0 |
u |
~ 0 |
% |
~ 0 |
39.582'000'000'0 |
MeV |
6.695'000'000'0 |
MeV |
- |
|
- |
|
4.75E-11 |
year |
1.500 |
milli-seconds ( x⁻³ ) |
100.000'000 |
% |
β- |
23,430.000 |
keV |
³⁴Mg |
³⁵Na > [ 100 % , β- , 23,430.0 keV ] > ³⁴Mg |
? |
% |
β-n |
22,702.000 |
keV |
³⁴Mg |
³⁵Na > [ ? % , β-n , 22,702.0 keV ] > ³⁴Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
66.856'000 |
% |
³⁴S |
26.904'000 |
% |
³⁵Cl |
6.500'000 |
% |
³³S |
? |
% |
³²S |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_36_u |
Unstable |
³⁶Na |
Fermion |
11 |
p |
25 |
n |
? |
0 |
36.051'480'000'0 |
u |
~ 0 |
% |
~ 0 |
47.953'000'000'0 |
MeV |
6.500'000'000'0 |
MeV |
- |
|
- |
|
8.24E-15 |
year |
260.000 |
nano-seconds ( x⁻⁹ ) |
? |
% |
n |
300.000 |
keV |
³⁵Mg |
³⁶Na > [ ? % , n , 300.0 keV ] > ³⁵Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
³⁴S |
? |
% |
³³S |
? |
% |
³²S |
? |
% |
³⁵Cl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_011_na_37_u |
Unstable |
³⁷Na |
Boson |
11 |
p |
26 |
n |
3/2 |
1 |
37.059'340'000'0 |
u |
~ 0 |
% |
~ 0 |
55.275'000'000'0 |
MeV |
6.345'000'000'0 |
MeV |
- |
|
- |
|
3.17E-17 |
year |
1.000 |
nano-seconds ( x⁻⁹ ) |
? |
% |
β- |
26,030.000 |
keV |
³⁷Mg |
³⁷Na > [ ? % , β- , 26,030.0 keV ] > ³⁷Mg |
? |
% |
β-n |
25,780.000 |
keV |
³⁵Mg |
³⁷Na > [ ? % , β-n , 25,780.0 keV ] > ³⁵Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
³⁶S |
? |
% |
³⁴S |
? |
% |
³⁷Cl |
? |
% |
³⁵Cl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|