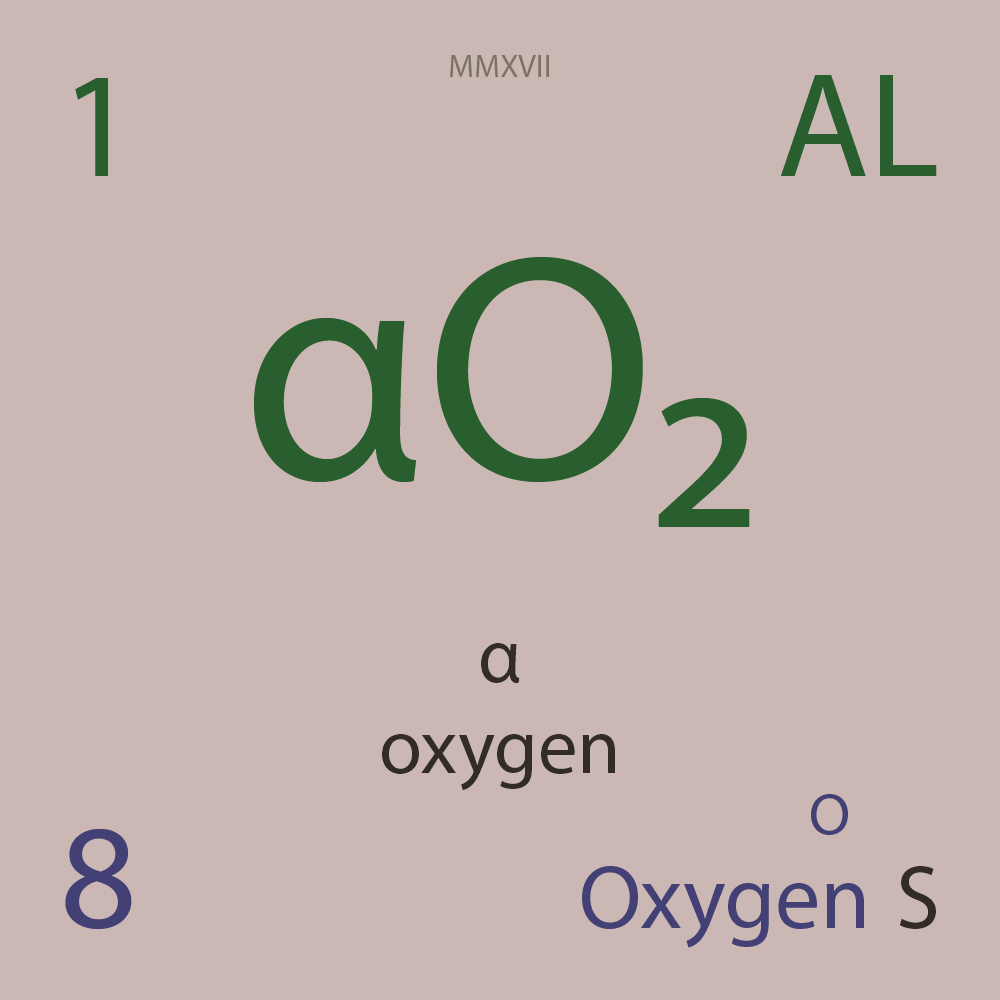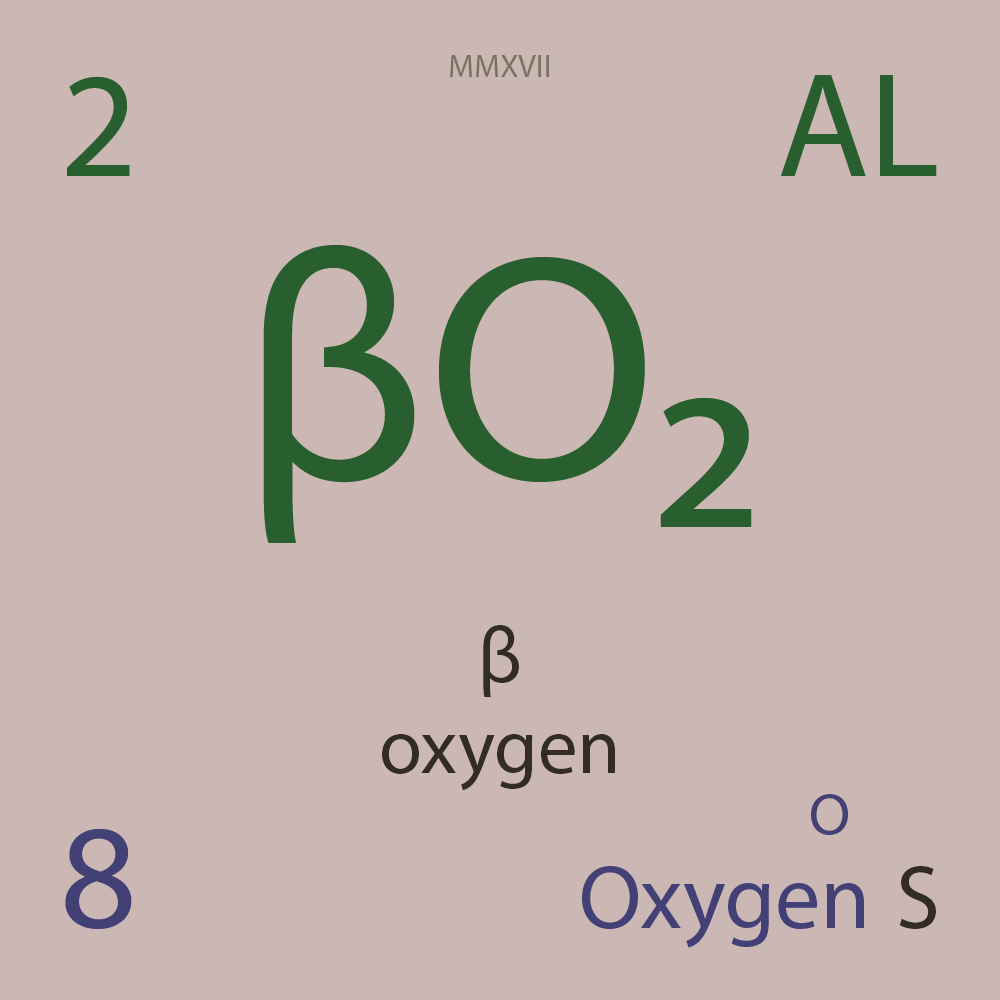| Isotope_008_o_12_u |
Unstable |
¹²O |
Boson |
8 |
p |
4 |
n |
0 |
1 |
12.034'404'895'0 |
u |
~ 0 |
% |
~ 0 |
32.047'954'000'0 |
MeV |
4.879'090'000'0 |
MeV |
- |
|
- |
|
1.84E-16 |
year |
5.800 |
nano-seconds ( x⁻⁹ ) |
60.000'000 |
% |
2p |
1,771.300 |
keV |
¹⁰C |
¹²O > [ 60 % , 2p , 1,771.3 keV ] > ¹⁰C |
? |
% |
β+ |
13,687.700 |
keV |
¹²N |
¹²O > [ ? % , β+ , 13,687.7 keV ] > ¹²N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
60.000'000 |
% |
¹⁰B |
? |
% |
⁴He |
? |
% |
¹²C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_13_u |
Unstable |
¹³O |
Fermion |
8 |
p |
5 |
n |
3/2 |
-1 |
13.024'812'213'0 |
u |
~ 0 |
% |
~ 0 |
23.112'428'000'0 |
MeV |
5.811'994'000'0 |
MeV |
- |
|
- |
|
2.72E-8 |
year |
858.000 |
milli-seconds ( x⁻³ ) |
89.000'000 |
% |
β+ |
16,744.750 |
keV |
¹³N |
¹³O > [ 89 % , β+ , 16,744.75 keV ] > ¹³N |
10.900'000 |
% |
β+p |
? |
keV |
¹²C |
¹³O > [ 10.9 % , β+p , ? keV ] > ¹²C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
89.000'000 |
% |
¹³C |
10.900'000 |
% |
¹²C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_14_u |
Unstable |
¹⁴O |
Boson |
8 |
p |
6 |
n |
0 |
1 |
14.008'596'250'0 |
u |
~ 0 |
% |
~ 0 |
8.007'356'000'0 |
MeV |
7.052'308'000'0 |
MeV |
- |
|
- |
|
2.24E-6 |
year |
70.598 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
4,121.739 |
keV |
¹⁴N |
¹⁴O > [ 100 % , β+ , 4,121.739 keV ] > ¹⁴N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁴N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_15_u |
Unstable |
¹⁵O |
Fermion |
8 |
p |
7 |
n |
1/2 |
-1 |
15.003'065'617'0 |
u |
~ 0 |
% |
~ 0 |
2.855'605'000'0 |
MeV |
7.463'692'000'0 |
MeV |
0.718'900'000'0 |
nm |
- |
|
3.87E-6 |
year |
122.240 |
seconds ( x⁰ ) |
100.000'000 |
% |
β+ |
1,731.966 |
keV |
¹⁵N |
¹⁵O > [ 100 % , β+ , 1,731.966 keV ] > ¹⁵N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁵N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_16_s |
Stable |
¹⁶O |
Boson |
8 |
p |
8 |
n |
0 |
1 |
15.994'914'619'6 |
u |
99.757'000 |
% |
15.956'046'977'0 |
-4.737'001'410'0 |
MeV |
7.976'206'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_17_s |
Stable |
¹⁷O |
Fermion |
8 |
p |
9 |
n |
5/2 |
1 |
16.999'131'703'0 |
u |
0.038'000 |
% |
0.006'459'670'0 |
-0.808'813'000'0 |
MeV |
7.750'731'000'0 |
MeV |
-1.893'790'000'0 |
nm |
-0.025'780'000'0 |
b |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_18_s |
Stable |
¹⁸O |
Boson |
8 |
p |
10 |
n |
0 |
1 |
17.999'161'001'0 |
u |
0.205'000 |
% |
0.036'898'280'1 |
-0.781'522'000'0 |
MeV |
7.767'025'000'0 |
MeV |
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_19_u |
Unstable |
¹⁹O |
Fermion |
8 |
p |
11 |
n |
5/2 |
1 |
19.003'580'130'0 |
u |
~ 0 |
% |
~ 0 |
3.334'870'000'0 |
MeV |
7.566'389'000'0 |
MeV |
- |
|
- |
|
8.39E-7 |
year |
26.464 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
4,822.260 |
keV |
¹⁹F |
¹⁹O > [ 100 % , β- , 4,822.26 keV ] > ¹⁹F |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
¹⁹F |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_20_u |
Unstable |
²⁰O |
Boson |
8 |
p |
12 |
n |
0 |
1 |
20.004'076'742'0 |
u |
~ 0 |
% |
~ 0 |
3.797'462'000'0 |
MeV |
7.568'505'000'0 |
MeV |
- |
|
- |
|
4.28E-7 |
year |
13.510 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
3,814.870 |
keV |
²⁰F |
²⁰O > [ 100 % , β- , 3,814.87 keV ] > ²⁰F |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²⁰Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_21_u |
Unstable |
²¹O |
Fermion |
8 |
p |
13 |
n |
? |
1 |
21.008'655'886'0 |
u |
~ 0 |
% |
~ 0 |
8.062'906'000'0 |
MeV |
7.389'332'000'0 |
MeV |
- |
|
- |
|
1.08E-7 |
year |
3.420 |
seconds ( x⁰ ) |
100.000'000 |
% |
β- |
8,110.500 |
keV |
²¹F |
²¹O > [ 100 % , β- , 8,110.5 keV ] > ²¹F |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.000'000 |
% |
²¹Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_22_u |
Unstable |
²²O |
Boson |
8 |
p |
14 |
n |
0 |
1 |
22.009'966'947'0 |
u |
~ 0 |
% |
~ 0 |
9.284'152'000'0 |
MeV |
7.364'821'000'0 |
MeV |
- |
|
- |
|
7.13E-8 |
year |
2.250 |
seconds ( x⁰ ) |
78.000'000 |
% |
β- |
6,490.800 |
keV |
²²F |
²²O > [ 78 % , β- , 6,490.8 keV ] > ²²F |
22.000'000 |
% |
β-n |
1,260.400 |
keV |
²¹F |
²²O > [ 22 % , β-n , 1,260.4 keV ] > ²¹F |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
69.420'000 |
% |
²²Ne |
30.580'000 |
% |
²¹Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_23_u |
Unstable |
²³O |
Fermion |
8 |
p |
15 |
n |
1/2 |
1 |
23.015'687'659'0 |
u |
~ 0 |
% |
~ 0 |
14.612'960'000'0 |
MeV |
7.163'850'000'0 |
MeV |
- |
|
- |
|
2.85E-9 |
year |
90.000 |
milli-seconds ( x⁻³ ) |
69.000'000 |
% |
β- |
11,283.000 |
keV |
²³F |
²³O > [ 69 % , β- , 11,283.0 keV ] > ²³F |
31.000'000 |
% |
β-n |
3,748.000 |
keV |
²²F |
²³O > [ 31 % , β-n , 3,748.0 keV ] > ²²F |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
59.339'000 |
% |
²³Ne |
37.250'000 |
% |
²²Ne |
3.409'990 |
% |
²¹Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_24_u |
Unstable |
²⁴O |
Boson |
8 |
p |
16 |
n |
0 |
1 |
24.020'472'917'0 |
u |
~ 0 |
% |
~ 0 |
19.070'400'000'0 |
MeV |
7.015'935'000'0 |
MeV |
- |
|
- |
|
2.06E-9 |
year |
65.000 |
milli-seconds ( x⁻³ ) |
82.000'000 |
% |
β- |
11,511.000 |
keV |
²⁴F |
²⁴O > [ 82 % , β- , 11,511.0 keV ] > ²⁴F |
18.000'000 |
% |
β-n |
7,669.000 |
keV |
²³F |
²⁴O > [ 18 % , β-n , 7,669.0 keV ] > ²³F |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
77.080'000 |
% |
²⁴Mg |
20.317'990 |
% |
²³Ne |
2.520'000 |
% |
²²Ne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_25_u |
Unstable |
²⁵O |
Fermion |
8 |
p |
17 |
n |
3/2 |
1 |
25.029'460'000'0 |
u |
~ 0 |
% |
~ 0 |
27.442'000'000'0 |
MeV |
6.723'000'000'0 |
MeV |
- |
|
- |
|
1.58E-15 |
year |
50.000 |
nano-seconds ( x⁻⁹ ) |
? |
% |
n |
300.000 |
keV |
²⁴O |
²⁵O > [ ? % , n , 300.0 keV ] > ²⁴O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
²⁵Na |
? |
% |
²⁴Ne |
? |
% |
²⁴Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_26_u |
Unstable |
²⁶O |
Boson |
8 |
p |
18 |
n |
0 |
1 |
26.038'340'000'0 |
u |
~ 0 |
% |
~ 0 |
35.713'000'000'0 |
MeV |
6.457'000'000'0 |
MeV |
- |
|
- |
|
1.27E-15 |
year |
40.000 |
nano-seconds ( x⁻⁹ ) |
70.000'000 |
% |
2n |
500.000 |
keV |
²⁵O |
²⁶O > [ 70 % , 2n , 500.0 keV ] > ²⁵O |
30.000'000 |
% |
n |
200.000 |
keV |
²⁵O |
²⁶O > [ 30 % , n , 200.0 keV ] > ²⁵O |
? |
% |
β- |
17,441.000 |
keV |
²⁶F |
²⁶O > [ ? % , β- , 17,441.0 keV ] > ²⁶F |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
53.956'000 |
% |
²⁵Mg |
14.222'600 |
% |
²⁵Na |
1.764'000 |
% |
²⁴Ne |
? |
% |
²⁶Mg |
? |
% |
²⁵Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_27_u |
Unstable |
²⁷O |
Fermion |
8 |
p |
19 |
n |
3/2 |
1 |
27.048'260'000'0 |
u |
~ 0 |
% |
~ 0 |
44.954'000'000'0 |
MeV |
6.175'000'000'0 |
MeV |
- |
|
- |
|
8.24E-15 |
year |
260.000 |
nano-seconds ( x⁻⁹ ) |
? |
% |
n |
1,170.000 |
keV |
²⁶O |
²⁷O > [ ? % , n , 1,170.0 keV ] > ²⁶O |
? |
% |
2n |
1,370.000 |
keV |
²⁵O |
²⁷O > [ ? % , 2n , 1,370.0 keV ] > ²⁵O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
²³Na |
? |
% |
²²Ne |
? |
% |
²⁶Mg |
? |
% |
²⁵Mg |
? |
% |
²⁴Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Isotope_008_o_28_u |
Unstable |
²⁸O |
Boson |
8 |
p |
20 |
n |
0 |
1 |
28.057'810'000'0 |
u |
~ 0 |
% |
~ 0 |
53.850'000'000'0 |
MeV |
5.925'000'000'0 |
MeV |
- |
|
- |
|
3.17E-15 |
year |
100.000 |
nano-seconds ( x⁻⁹ ) |
? |
% |
β- |
20,623.000 |
keV |
²⁸F |
²⁸O > [ ? % , β- , 20,623.0 keV ] > ²⁸F |
? |
% |
2n |
1,994.000 |
keV |
²⁶O |
²⁸O > [ ? % , 2n , 1,994.0 keV ] > ²⁶O |
? |
% |
n |
824.000 |
keV |
²⁷O |
²⁸O > [ ? % , n , 824.0 keV ] > ²⁷O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
? |
% |
²³Na |
? |
% |
²²Ne |
? |
% |
²⁶Mg |
? |
% |
²⁵Mg |
? |
% |
²⁴Mg |
? |
% |
²⁷Al |
? |
% |
²⁷Al |
? |
% |
²⁷Al |
? |
% |
²⁷Al |
? |
% |
²⁷Al |
? |
% |
²⁷Al |
? |
% |
²⁷Al |