HYDROGEN, H (1)
HYDROGEN TO OGANESSON
ATOMIC ARCHITECTURE
HYDROGEN
H (1)
ˈhaɪdrədʒən
PHONETICS
Hydrogen, the lightest chemical element, is a diatomic gas that is colourless, odourless, and flammable at room temperature.
ELEMENT BRIEF
1766, Henry Cavendish, England.
DISCOVERY
Ancient Greek, ὕδωρ (húdōr, 'water')
+ γεννάω (gennáō, 'I bring forth').
ETYMOLOGY
Hydrogen
ELEMENT
H
SYMBOL
1
ATOMIC NUMBER
1333-74-0
CAS NUMBER
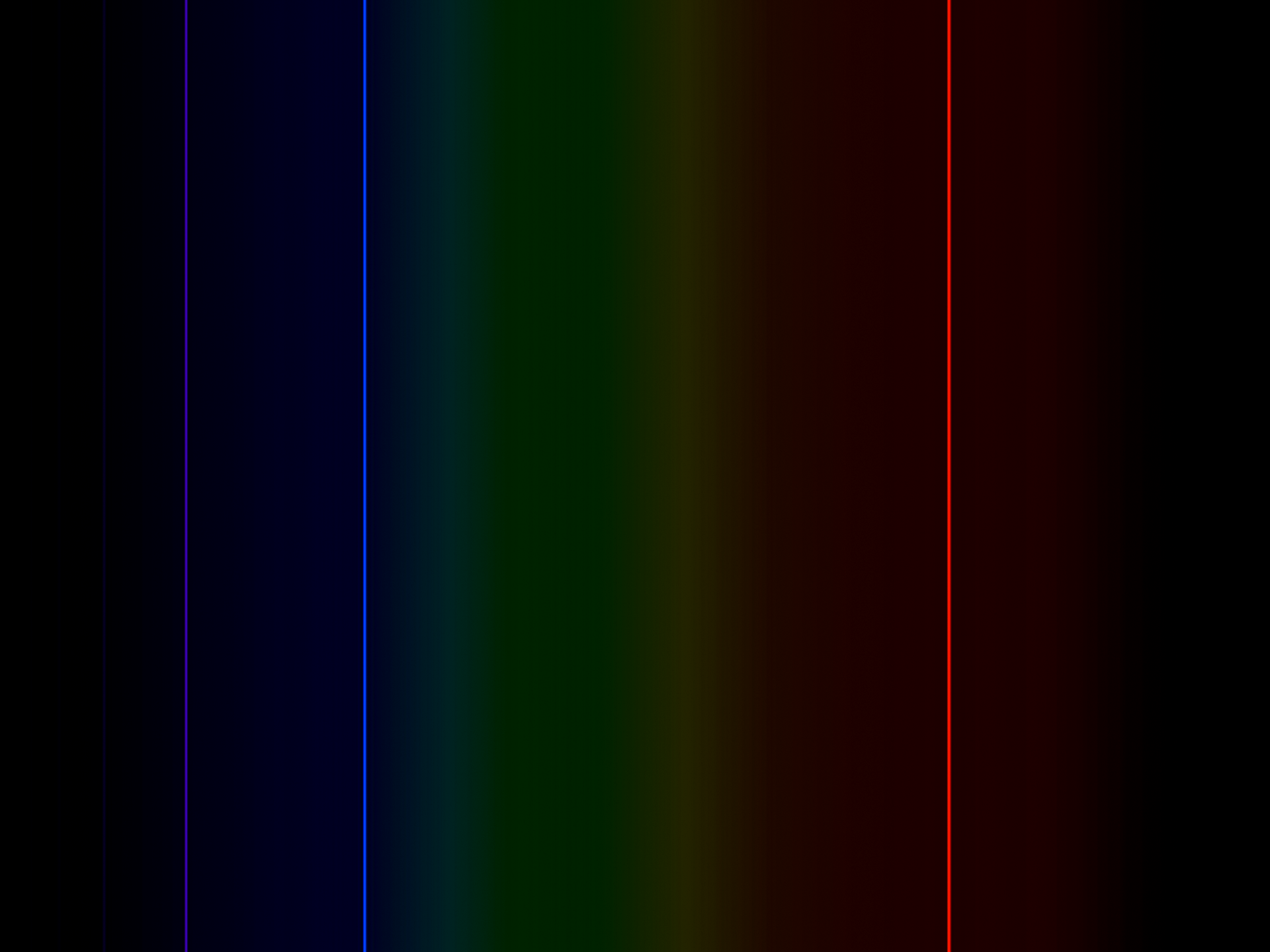
ELEMENTAL SPECTRUM
Colourless
ELEMENTAL COLOUR
1.000'132
REFRACTIVE INDEX
-
POISSON RATIO
11.214 cm³
MOLAR VOLUME
-
BRINELL HARDNESS
-
MOHS HARDNESS
-
VICKERS HARDNESS
1,270 m/s, Mach 3.702'6
SPEED OF SOUND
-
BULK MODULUS
-
SHEAR MODULUS
-
YOUNG MODULUS
ALLOTROPES
 |
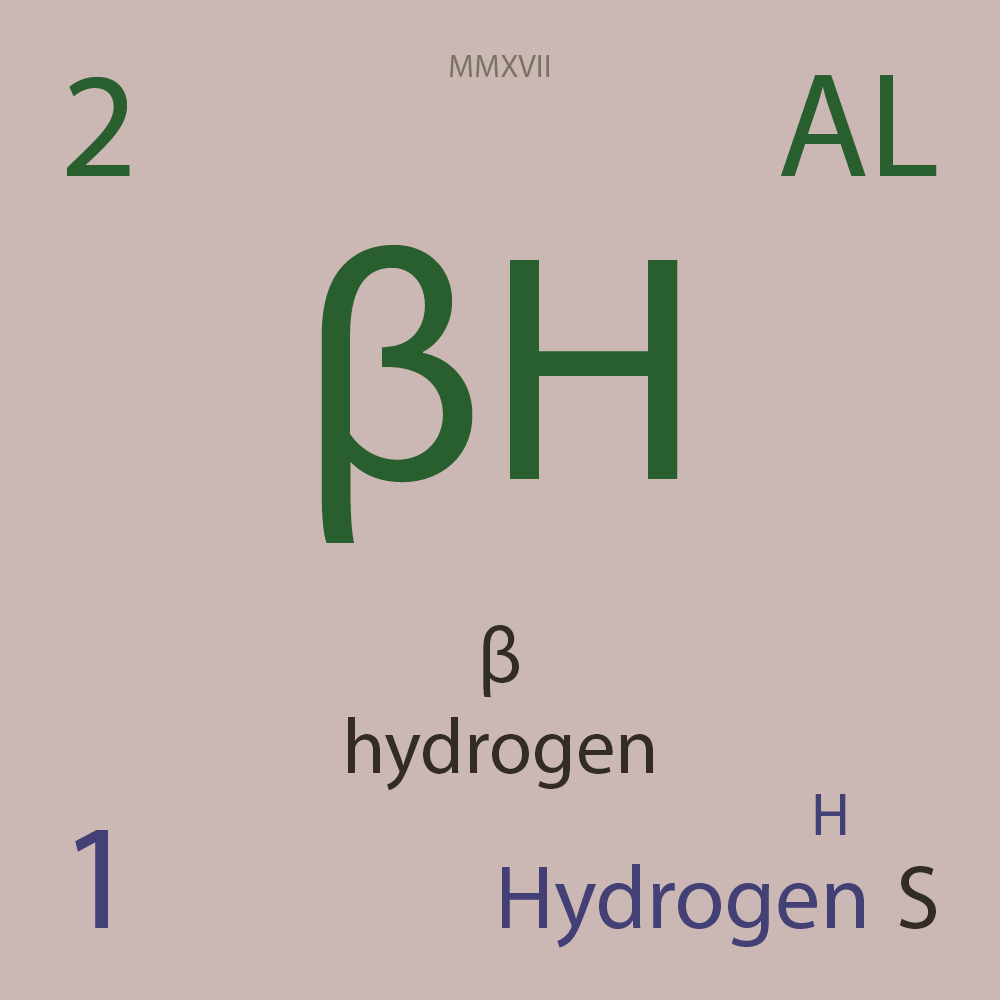 |
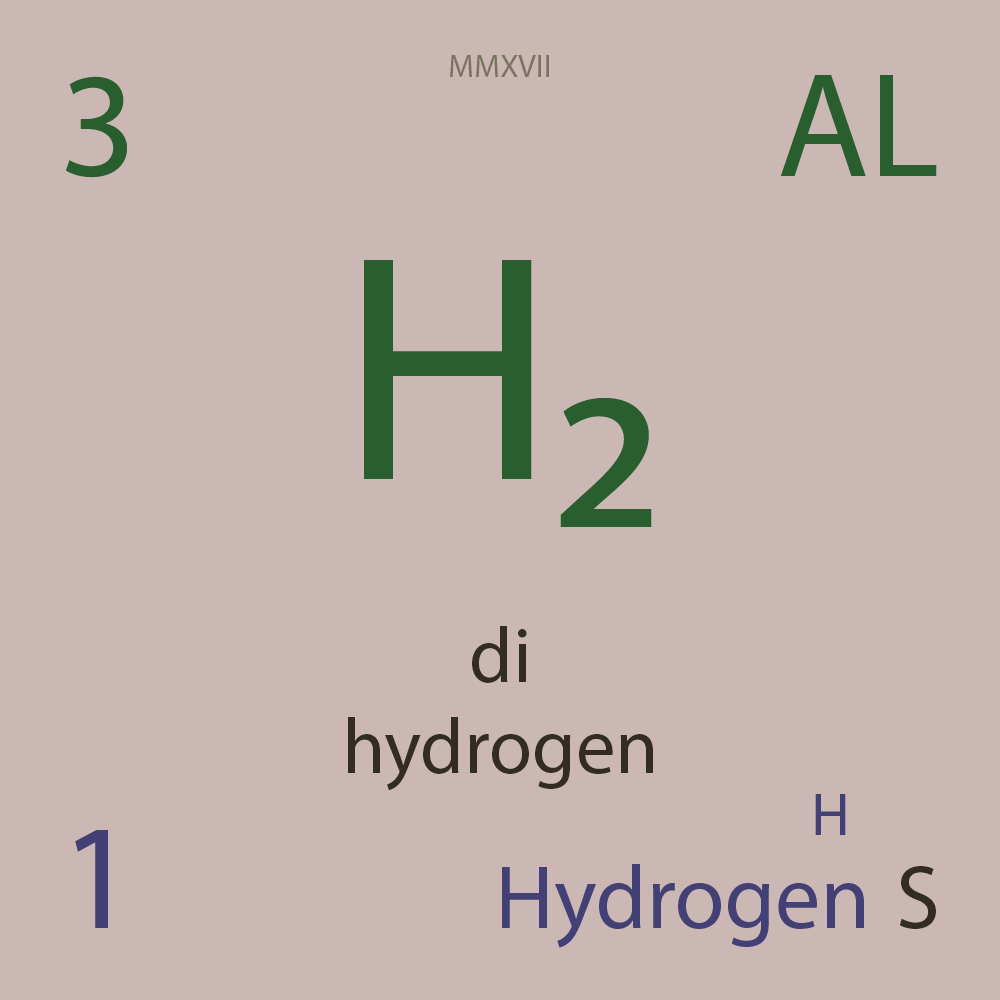 |
14.01 K, -259.14 °C
MELTING POINT
20.28 K, -252.87 °C
BOILING POINT
808.65 K, 535.50 °C
AUTOMATIC IGNITION
255.15 K, -18.00 °C
FLASHPOINT
32.97 K, -240.18 °C
CRITICAL TEMPERATURE
1.29M Pa, 13 Atm
CRITICAL PRESSURE
0.180'500 W/(m K)
THERMAL CONDUCTIVITY
-
THERMAL EXPANSION
14,300.00 J/(kg K)
SPECIFIC HEAT
0.558 kJ/mol
HEAT FUSION
0.452 kJ/mol
HEAT VAPORISATION
-
HEAT COMBUSTION
-
CURIE POINT
-
NEEL POINT
7/5
ADIABATIC INDEX
Gas
PHASE
-
ELECTRICAL TYPE
-
CONDUCTIVITY
-
RESISTIVITY
-
SUPERCONDUCTING POINT
Diamagnetic
MAGNETIC TYPE
-0.000'000'024'8
MASS SUSCEPTIBILITY
-0.000'000'000'049'99
MOLAR SUSCEPTIBILITY
-0.000'000'002'23
VOLUME SUSCEPTIBILITY
Non Metal
CLASSIFICATION
1.007'940'8
ATOMIC WEIGHT
53 pm
ATOMIC RADIUS
32 pm
COVALENT RADIUS SINGLE BOND
-
COVALENT RADIUS DOUBLE BOND
-
COVALENT RADIUS TRIPLE BOND
110 pm
VAN DER WAALS RADIUS
1s¹
ELECTRON CONFIGURATION
Hexagonal, Primitive
CRYSTAL STRUCTURE
-
DENSITY AS SOLID
-
DENSITY AS LIQUID
0.089'88 g/cm³
DENSITY AS GAS
P6₃/mmc
SPACE GROUP NAME
194
SPACE GROUP NUMBER
π/2, π/2, 2π/3
LATTICE ANGLES
470, 470, 340 pm
LATTICE CONSTANTS
1
VALENCE
2.2
ELECTRONEGATIVITY
72.77 kJ/mol
ELECTRON AFFINITY
IONISATION ENERGY
 |
75 %
UNIVERSE
2.4 %
METEORITES
75 %
SUN
0.154 %
EARTH CRUST
10.623 %
OCEANS
10.172 %
HUMANS
Stable
HALF LIFE
Stable
LIFETIME
1s¹ = 1, 0, 0, +1/2
QUANTUM NUMBERS
82.020'00 b σs
NEUTRON CROSS SECTION
0.332'00 b σa
NEUTRON MASS ABSORPTION
STABLE ISOTOPES
 |
 |
UNSTABLE ISOTOPES
 |
 |
 |
 |
 |
ISOTOPIC CHAIN
CENTRALDATACORE
SHARE

